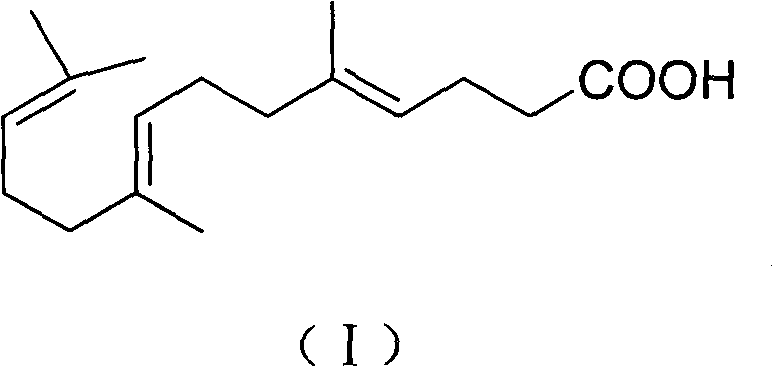
New preparation method for gefarnate key intermediate
A technology of gefar esters and intermediates, which is applied in the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems that are not suitable for industrial production, achieve simple operation, mild reaction conditions, and reduce energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]Add 110g of intermediate (III) into a 1000ml three-neck flask, add 202g of potassium carbonate, 1.6g of sodium iodide, 76g of diethyl malonate, and 200ml of acetone, and heat up to reflux reaction (internal temperature 60-70°C), TLC Track the completion of the reaction (developing agent: ethyl acetate:petroleum ether=1:9; iodine color development) for about 4 hours. After the reaction is complete, acetone is recovered under normal pressure, and 200ml of purified water is added to the reaction bottle and stirred until the potassium carbonate is dissolved, and the water is separated. Layer, collect the organic layer, combine the organic layer, dry with anhydrous sodium sulfate, filter with suction, remove the fraction before 100°C from the organic layer under reduced pressure, and the residue is intermediate (IV) 146.3g.
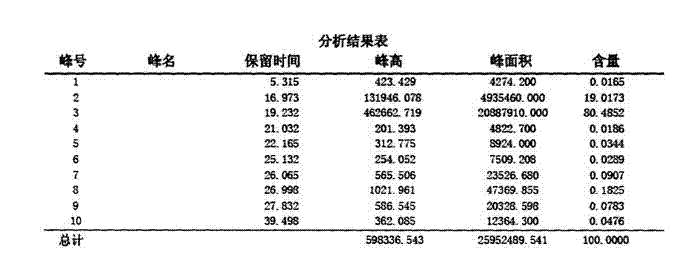
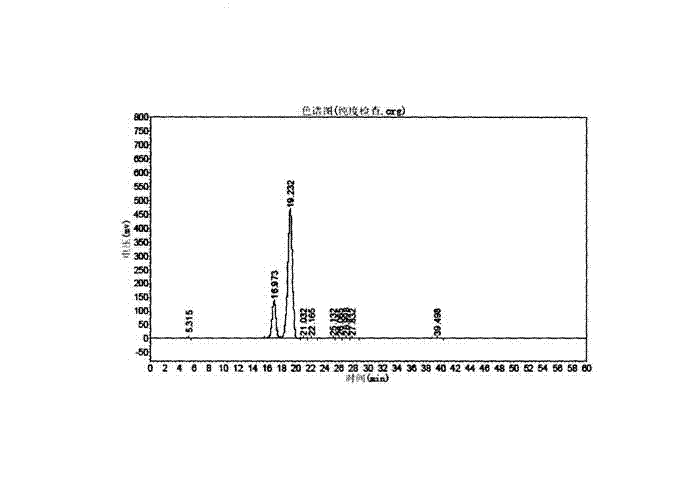
[0042] Gefarate purity inspection method: high performance liquid chromatography-evaporative light scattering detection (HPLC-ELSD) method, chromatograph...
Embodiment 2
Embodiment 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com