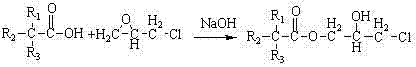Method for preparing versatate glycidyl
A technology of glycidyl ester and tertiary carbonic acid is applied in the field of preparation of glycidyl tertiary carbonate, can solve the problems of low yield of glycidyl tertiary carbonate and high preparation cost, and achieves a product with good product quality, low preparation cost and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
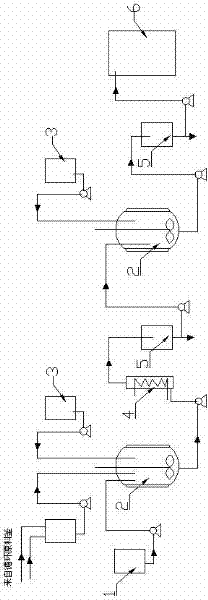
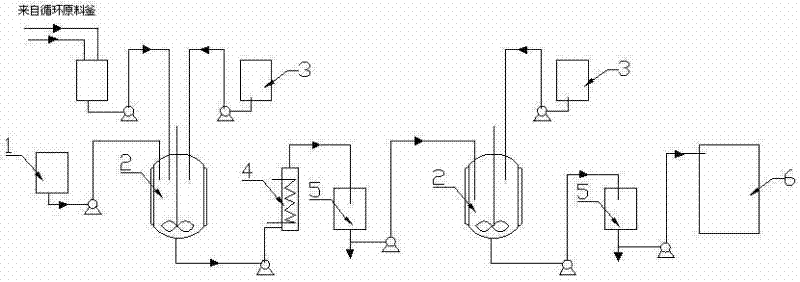
[0037] Embodiment 1: as figure 1 Shown, a kind of preparation method of glycidyl tertiary carbonate comprises the following steps:
[0038] A. Add 100g of ethanol, 100g of water and 69.4g of epichlorohydrin to a four-necked 1L flask with stirring, thermometer and reflux, heat to 55°C, and mix 129.3g of tertiary carbonic acid and 12.3g of sodium hydroxide solution ( Sodium hydroxide (50% by weight) was added dropwise in the flask, and the feeding time was controlled to be 20 minutes, and the reaction was carried out at 55° C. for 2.0 hours;
[0039] B. Cool the reaction mixture obtained in step A to 40°C, separate the water phase, add 24g of sodium hydroxide and 74g of water, and react at 40°C for 80 minutes;
[0040] C. Distill the reaction mixture obtained in step B at a pressure of 5KPa and a temperature of 120°C, then add 20mL of water for stripping, keep the pressure at 5KPa, and the temperature is 140°C for 10 minutes to remove excess epichlorohydrin , solvent and the w...
Embodiment 2
[0042] Embodiment 2: as figure 1 Shown, a kind of preparation method of glycidyl tertiary carbonate comprises the following steps:
[0043] A. Add 400g of isopropanol, 605g of water and 300g of epichlorohydrin to a four-necked 2L flask with stirring, thermometer and reflux, heat to 80°C, mix 500g of tertiary carbonic acid and 47.6g of sodium hydroxide solution ( Sodium hydroxide weight percentage is 50%) dropwise in the flask, and feed time is controlled as 20 minutes, and reaction is carried out 100 minutes under 80 ℃ of conditions;
[0044] B. Cool the reaction mixture obtained in step A to 60°C, separate the water phase, add 93g of sodium hydroxide and 280g of water, and then react at 60°C for 100 minutes;
[0045]C. Distill the reaction mixture obtained in step B at a pressure of 95.4KPa and a temperature of 120°C, and then continue to distill under reduced pressure until the pressure is 5KPa and the temperature is 140°C to remove excess epichlorohydrin, solvent and react...
Embodiment 3
[0047] Embodiment 3: as figure 1 Shown, a kind of preparation method of glycidyl tertiary carbonate comprises the following steps:
[0048] A. Add 124.9g of tert-butanol, 100g of water and 234.9g of epichlorohydrin to a four-necked 1L flask with stirring, thermometer and reflux, heat to 65°C, and oxidize 129.3g of tertiary carbonic acid and 12.3g of hydrogen Sodium solution (sodium hydroxide weight percent is 50%) is added dropwise in the flask, and the feeding time is controlled as 20 minutes, and the reaction is carried out at 65° C. for 30 minutes;
[0049] B. Cool the reaction mixture obtained in step A to 55° C., separate the water phase, add 24.0 g of potassium hydroxide and 74 g of water, and react at 55° C. for 30 minutes;
[0050] C. Distill the reaction mixture obtained in step B at a pressure of 95.4KPa and a temperature of 120°C, and then continue to distill under reduced pressure until the pressure is 5KPa and the temperature is 140°C to remove excess epichlorohy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy equivalent value | aaaaa | aaaaa |
| Epoxy equivalent value | aaaaa | aaaaa |
| Epoxy equivalent value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com