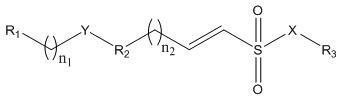
2-substituted vinylsulfonate compound and preparation method and use thereof
A technology for ethylene sulfonate and compound, which is applied in the field of 2-substituted ethylene sulfonate compound and its preparation, and can solve problems such as large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
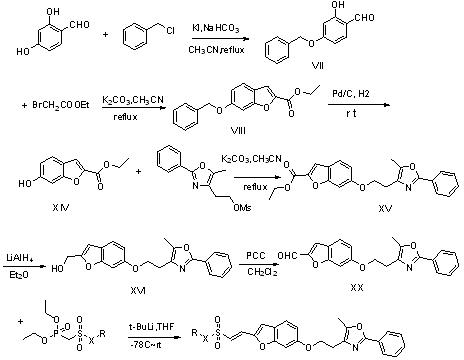
Image
Examples
Embodiment 1
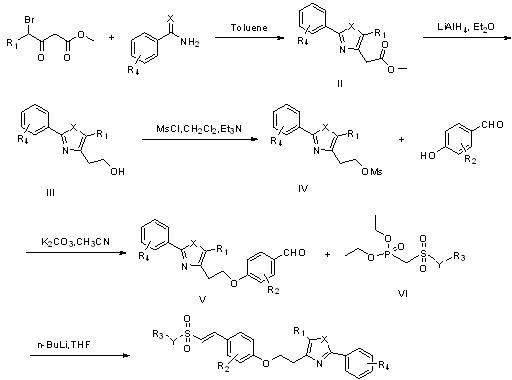
[0058] Synthesis of 2-(5-methyl-2-phenyloxazol-4-yl)methyl acetate II ( figure 1 ): Dissolve methyl 4-bromo-3-oxopentanoate (10g, 45mmol) in toluene (200ml), then add benzamide (5.45g, 45mmol) in batches, and reflux for 12 hours after the addition. After the reaction was completed, it was filtered and concentrated under reduced pressure, and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain methyl 2-(5-methyl-2-phenyloxazol-4-yl)acetate 4.4 g (yellow oil, yield 40%). 1 H NMR ((CD 3 ) 2 CO; 300MHz), δ H : 2.378 (s, 3H, CH 3 ), 3.587 (s, 2H, CH 2 ), 3.665 (s, 3H, OCH 3 ), 7.473-7.499 (m, 3H, ArH), 7.950-7.982 (m, 2H, ArH).
Embodiment 2
[0060] Synthesis of 2-(5-methyl-2-phenyloxazol-4-yl)ethanol III ( figure 1 ): Dissolve lithium aluminum tetrahydride (207.1mg, 5.45mmol) in anhydrous ether (20ml), add 2-(5-methyl-2-phenyloxazol-4-yl) dropwise at -5°C Diethyl ether solution of methyl acetate (890mg, 3.63mmol), after the dropwise addition, was stirred at room temperature for half an hour. After the reaction was completed, a saturated ammonium chloride aqueous solution was added dropwise to the reaction system to quench until white flocs appeared in the reaction system. Filter, wash the aqueous phase with ethyl acetate, combine the organic phases, wash with saturated brine three times, and dry over anhydrous sodium sulfate. Concentration under reduced pressure gave 710 mg of 2-(5-methyl-2-phenyloxazol-4-yl)ethanol (colorless solid, yield 96%). 1 H NMR (CDCl 3 ; 300MHz), δ H : 2.347 (s, 3H, CH 3 ), 2.754-2.792 (t, 2H, CH 2 CH 2 , J=5.7Hz), 3.924-3.963 (t, 2H, CH 2 CH 2 , J=5.8Hz), 6.0-6.5 (brs, 1H, O...
Embodiment 3
[0062] Synthesis of ethyl 2-(5-methyl-2-phenyloxazol-4-yl)methanesulfonate IV ( figure 1 ): Dissolve 2-(5-methyl-2-phenyloxazol-4-yl)ethanol (630mg, 3.1mmol) in dichloromethane (15ml), drop triethylamine (0.64ml, 4.65mmol ), and then methanesulfonyl chloride (0.37ml, 4.65mmol) was added dropwise to the reaction system at 0°C, and stirred at room temperature for 4 hours after the dropwise addition was completed. After the reaction was completed, saturated ammonium chloride aqueous solution was added dropwise to the reaction system to quench, the aqueous phase was washed with ethyl acetate, the organic phases were combined, washed three times with saturated brine, and dried over anhydrous sodium sulfate. Concentrated under reduced pressure and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 700 mg of ethyl 2-(5-methyl-2-phenyloxazol-4-yl)methanesulfonate (colorless Solid, yield 80%). 1 H NMR (CDCl 3 ; 300MHz), δ H : 2.365 (s, 3H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com