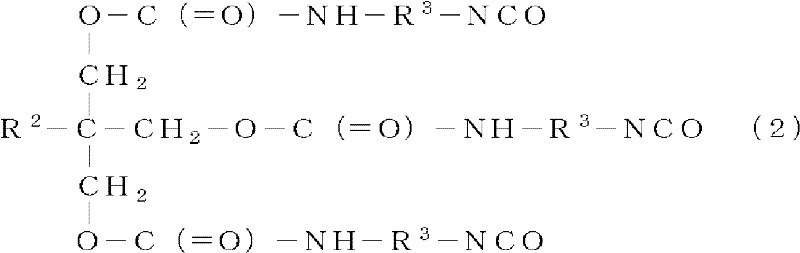Non-water-based for plastic film provided with active energy line curing film and plastic film provided with active energy line curing film
A technology of active energy lines and plastic films, applied in polyester coatings, chemical instruments and methods, polyurea/polyurethane coatings, etc., can solve the problems of decreased adhesion, poor moisture resistance of the primer layer, and obtaining primer films, etc. Achieve excellent dryness and moisture resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0055] Production example 1
[0056] In a reaction vessel with a mixer, cooler, thermometer, and nitrogen inlet tube, add 479 parts of terephthalic acid, 402 parts of isophthalic acid, 87 parts of azelaic acid, 256 parts of ethylene glycol, 221 parts of 1,6- Hexylene glycol, 148 parts of neopentyl glycol and 7 parts of trimethylolpropane were heated to melt the reaction system with stirring. Then, the water produced in the dehydration condensation reaction was removed, and the temperature of the reaction system was slowly raised from 160°C to 200°C within 3 hours, and then kept at 200°C for 1 hour. Next, 0.16 parts of antimony trioxide was added. Then, a vacuum decompression device was connected to the reaction container, and a reduced-pressure compression polymerization reaction was carried out at 235° C. and 2.8 kPa for 1 hour. Then, 512 parts of methyl isobutyl ketone and 768 parts of methyl ethyl ketone were added and dissolved uniformly. Thereby, the solution of polye...
manufacture example 2-11、 comparative manufacture example 1
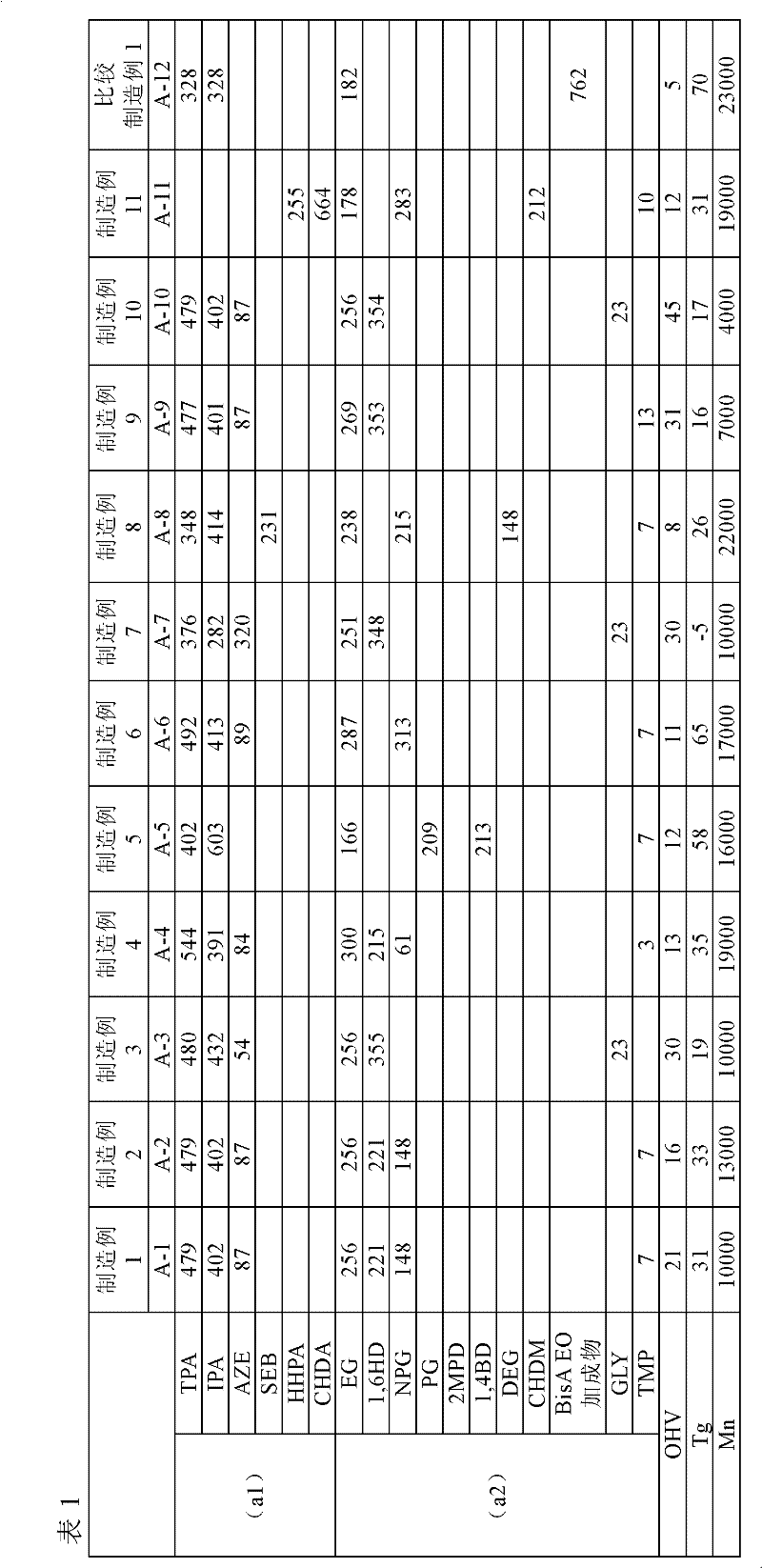
[0058] Except that the type and usage amount of the (a1) component-(a3) component are changed to the contents shown in Table 1, other is the same as Production Example 1 to obtain branched polyester resins (A-2)-(A-11 ) and solutions of unbranched polyester resin (A-12). In addition, the unit of the usage-amount of each component in Table 1 is a part.
[0059]
[0060] In Table 1, each symbol has the following meanings.
[0061] TPA: Terephthalic acid
[0062]IPA: Isophthalic acid
[0063] AZE: Azelaic acid
[0064] SEB: Sebacic acid
[0065] HHPA: Hexahydrophthalic anhydride
[0066] CHDA: 1,4-cyclohexanedicarboxylic acid
[0067] EG: ethylene glycol
[0068] 1,6HD: 1,6-Hexanediol
[0069] NPG: Neopentyl glycol
[0070] PG: propylene glycol
[0071] 2MPD: 2-methyl-1,3-propanediol
[0072] 1,4BD: 1,4-butanediol
[0073] DEG: diethylene glycol
[0074] CHDM: 1,4-Cyclohexanedimethanol
[0075] BisAEO adduct: Ethylene oxide adduct of bisphenol A
[0076] GLY: Gl...
Embodiment 1
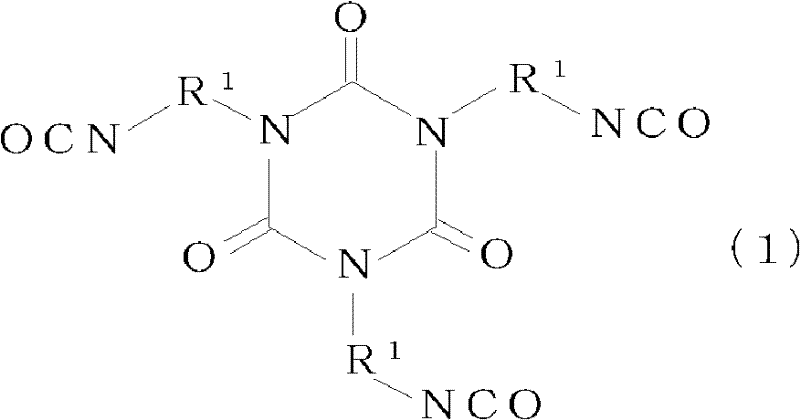
[0083] To 100 parts of the solution of the branched polyester resin (A-1) obtained in Production Example 1, 1.5 parts of pentaerythritol triacrylate (trade name "Biscoat #300": Osaka Organic Co., Ltd. Chemical Industry Co., Ltd.), mixing. Then, 8.9 parts of "Coronet HX" (uric acid body of hexamethylene diisocyanate (trade name: Nippon Polyuretan Industries, Ltd.)) as component (C) and 0.25 parts as (D) were added to this solution. Dioctyltin dilaurate as a component (trade name: "Neostan U-810": Nitto Kasei Co., Ltd.) was diluted with 131 parts of methyl ethyl ketone as component (E) to obtain a primer with a non-volatile content of 25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com