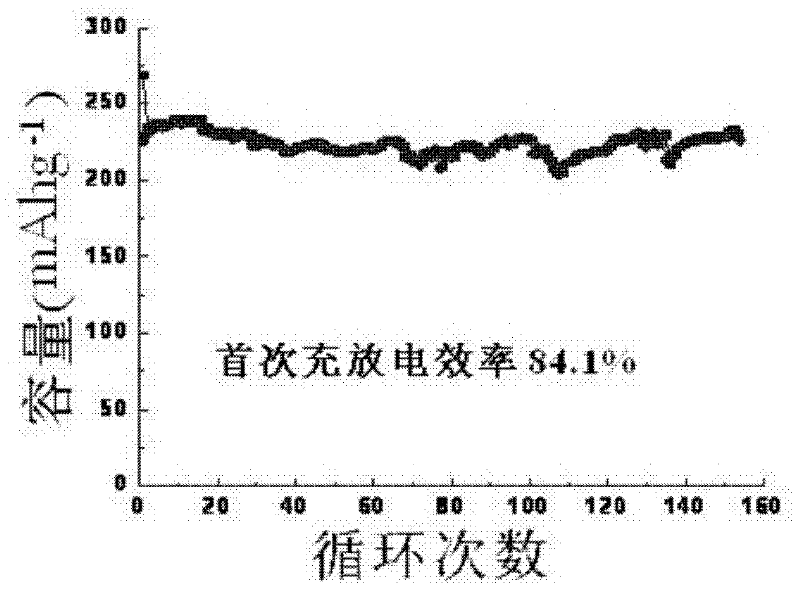
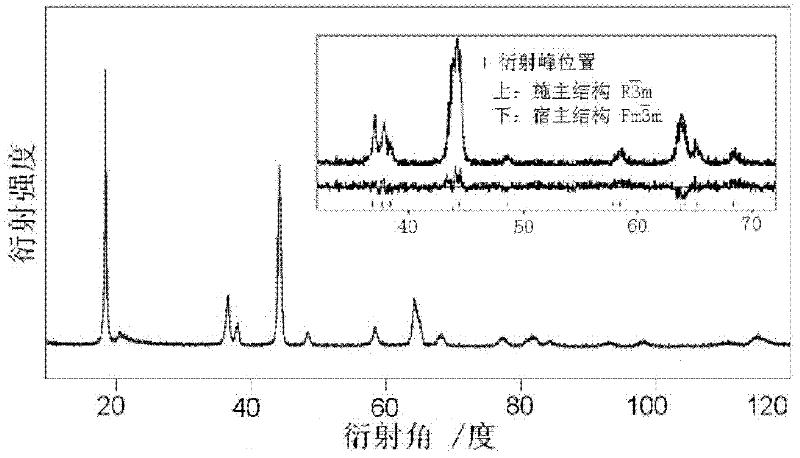
Preparation method of layered manganese-based cathode material for raising initial charge/discharge efficiency
A technology of charge-discharge efficiency and positive electrode material is applied in the field of preparation of positive electrode active materials for lithium-ion batteries, which can solve the problems of phase composition control of transition metal ions, deviation of transition metal ion ratio from stoichiometry, waste of raw materials, etc., so as to improve the first charge-discharge efficiency. , Precise control of chemical composition, improved charge-discharge efficiency and cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Weigh nickel nitrate and manganese nitrate with a molar ratio of 0.75:1.25 to prepare a 1mol / l mixed solution, use ammonium bicarbonate as a precipitant, and add ammonia water dropwise at the same time to control the pH value to 8, and coprecipitate for 10 hours.
[0029] 2) Filter out the co-precipitation product, add deionized water to prepare a slurry with a concentration of 180g / l, and spray dry the slurry at 115°C to obtain nickel-manganese carbonate powder;
[0030] 3) Grinding the nickel-manganese carbonate powder, incubating at 300°C for 5.5 hours, then raising the temperature to 450°C for 2 hours to obtain the precursor powder;
[0031] 4) Weigh lithium hydroxide monohydrate with a molar number of 1.02 (K[1+x / (2+x)], where K=0.92), grind and mix with the precursor powder obtained above, and roast at 800°C for 8 hour, synthesize 0.25Li 2 MnO 3 0.75LiNi 1 / 2 mn 1 / 2 o 2 (xLi 2 MnO 3 ·(1-x)LiNi 1 / 2 mn 1 / 2 o 2 , where x=0.25) powder;
[0032] 5) 0.25Li...
Embodiment 2
[0035] 1) Weigh nickel nitrate and manganese nitrate with a molar ratio of 0.6:1.4 to prepare a 1.5mol / l mixed solution, use ammonium bicarbonate as a precipitant, and add ammonia water dropwise at the same time, control the pH value to 9, and co-precipitate for 8 hours.
[0036] 2) Filter out the co-precipitation product, add deionized water to prepare a slurry with a concentration of 150g / l, and spray dry the slurry at 120°C to obtain nickel-manganese carbonate powder;
[0037] 3) Grinding the nickel-manganese carbonate powder, incubating at 320°C for 4 hours, then raising the temperature to 500°C for 3 hours to obtain the precursor powder;
[0038] 4) Weigh lithium hydroxide monohydrate with a molar number of 1.11 (K[1+x / (2+x)], where K=0.95), grind and mix with the precursor powder obtained above, and roast at 850°C for 10 hour, synthesize 0.4Li 2 MnO 3 0.6LiNi 1 / 2 mn 1 / 2 o 2 (xLi 2 MnO 3 ·(1-x)LiNi 1 / 2 mn 1 / 2 o 2 , where x=0.4) powder;
[0039] 5) 0.4Li 2 MnO...
Embodiment 3
[0041] 1) Weigh nickel nitrate and manganese nitrate with a molar ratio of 0.7:1.3 to prepare a 1.2mol / l mixed solution, use ammonium bicarbonate as a precipitant, and add ammonia water dropwise at the same time, control the pH value to 8.5, and co-precipitate for 12 hours.
[0042] 2) Filter out the co-precipitation product, add deionized water to prepare a slurry with a concentration of 200g / l, and spray dry the slurry at 110°C to obtain nickel-manganese carbonate powder;
[0043] 3) Grinding nickel-manganese carbonate powder, incubating at 300°C for 4 hours, then raising the temperature to 480°C for 2 hours to obtain precursor powder;
[0044] 4) Weigh lithium hydroxide monohydrate with a molar number of 1.03 (K[1+x / (2+x)], where K=0.91), grind and mix with the precursor powder obtained above, and roast at 850°C for 10 hour, synthesize 0.3Li 2 MnO 3 0.7LiNi 1 / 2 mn 1 / 2 o 2 (xLi 2 MnO 3 ·(1-x)LiNi 1 / 2 mn 1 / 2 o 2 , where x=0.3) powder;
[0045] 5) 0.3Li 2 MnO 3 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com