Clone and application of pig skeletal muscle specificity expression gene alpha-actin promoters
A skeletal muscle-specific gene expression technology, applied in the field of functional verification, can solve the problems of α-actin promoter function of skeletal muscle gene in pig muscle tissue, lack of targeted expression of regulatory genes, and low specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Obtaining candidate promoter fragments and corresponding deletion fragments of porcine skeletal muscle-specific expression gene α-actin
[0036] The DNA sequence of the reported porcine α-actin gene (GenBank accession number: 100154254 ) is the probe sequence, and the 2006 bp upstream sequence before the candidate gene ATG was extracted from NCBI (http: / / www.ncbi.nlm.nih.gov / ) as a candidate promoter fragment. By designing specific primers (P1F: CCATCTGGAGGAAGCACG; P1R: CCACGATGGACGGGAACA), and using the blood genomic DNA of large white pigs as a template, the sequence was amplified by PCR. The amplification conditions were: 94°C for 5 min, 35* (94°C, 40sec, 57.8°C, 40sec, 72°C, 1min30sec); 72°C, 10min; 25°C, 2min. The amplified product was detected by 1.5% agarose electrophoresis, recovered and purified (operated by the UNIQ-10 column DNA gel recovery kit of Beijing Boda Tech Bio-Gene Technology Co., Ltd.), and TA cloning was performed. The ligation system ...
Embodiment 2
[0043] Example 2: Construction of the transfection vector of the promoter candidate fragment of the porcine skeletal muscle-specific expression gene α-actin and the corresponding deletion fragment.
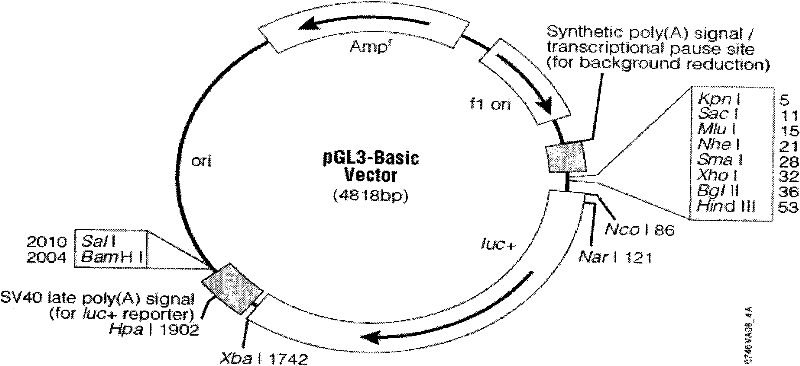
[0044] (1) The enzyme-digested vector pGL3-basic (the vector was purchased from Promega (Beijing) Biotechnology Co., Ltd., that is, Promega Corporation in the United States. For information on the vector and multiple cloning sites, see figure 2 ) and the TA cloning plasmids TA-Q1, TA-Q2, TA-Q3, TA-Q4, TA-Q5, TA-Q6, TA-Q7, TA-Q8 with deletion fragments. Use KpnI, BglII double enzyme digestion pGL3-basic and TA-Q1, TA-Q2, TA-Q3, TA-Q4, TA-Q5, TA-Q6, TA-Q7, TA-Q8, the digestion system is:
[0045] 10×buffer2 2μL;
[0047] 100× bovine serum albumin (BSA) 0.2 μL;
[0048] Kpn I 0.5 μL;
[0049] BglII 0.5 μL;
[0050] Double distilled water 11.8μL
[0051]After digestion at 37°C for 3 hours, use 1.5% agarose gel electrophoresis to check the integrity of ...
Embodiment 3
[0059] Example 3: Liposome-mediated cell transfection
[0060] In the present invention, a 24-well cell culture dish is used for transfection. In order to eliminate experimental errors, three independent experiments were performed for each recombinant vector, and three replicate wells were performed for each experiment. According to Lipofectamine TM 2000 (invitrogen company) instructions, each well was transfected according to the ratio of the mass of the carrier: the volume of the liposome = 1 μg: 3 μL. The transfected receptors were cells from different sources, including mouse muscle cell line C2C12, porcine kidney cell PK and myotubes formed by the differentiation of C2C12 cells induced by 2% horse serum. 48h after the recombinant vector was transfected into the cells, the cell lysate was collected, and the activity of the missing promoter was analyzed by the dual luciferase detection system. The main steps of cell culture, induced differentiation and transfection of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com