Preparation method of lincomycin hydrochloride injection
A technology for lincomycin hydrochloride and injection, which is applied in the directions of medical preparations, pharmaceutical formulations, and drug delivery containing active ingredients, can solve problems such as increasing manufacturing costs, unfavorable environmental protection, etc., and achieves reducing pain and improving quality. , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Weigh 6kg of lincomycin hydrochloride original powder, 8g of EDTA-2Na, 172g of anhydrous sodium bicarbonate, and about 19000ml of water for injection.
[0017] a. Heat the water for injection to 60°C, dissolve the original powder of lincomycin hydrochloride, adjust the pH value of the liquid to 4.1-4.5 with hydrochloric acid, make the liquid acidic, stir well, and mix it with lincomycin The mixed alcohol residual reaction, then add water for injection to 20000ml.
[0018] b. Use 5000 Dalton ultrafiltration equipment for ultrafiltration, and add formula amount of anhydrous sodium bicarbonate to the solution after ultrafiltration to reduce irritation and pain during administration, add EDTA-2Na, and stir well Finally, depyrogenate with 0.02% activated carbon for needles, measure content and pH value.
[0019] c. After the drug solution is qualified, it is filtered through a 0.2μm microporous membrane, filled in a 100-grade environment, sterilized once by circulating stea...
Embodiment 2
[0022] Weigh 3kg of lincomycin hydrochloride original powder, 6g of EDTA-2Na, appropriate amount of anhydrous sodium bicarbonate, and about 19000ml of water for injection.
[0023] a. Heat the water for injection to 60°C, dissolve the original powder of lincomycin hydrochloride, adjust the pH value of the liquid to 4.5-5.5 with hydrochloric acid, make the liquid acidic, stir well, and mix it with lincomycin The mixed alcohol residual reaction, then add water for injection to 20000ml.
[0024] b. Use 5000 Dalton ultrafiltration equipment for ultrafiltration, add appropriate amount of anhydrous sodium bicarbonate to the solution after ultrafiltration to adjust the pH value to neutral, add EDTA-2Na, stir well and use 0.02% activated carbon for needles to remove Pyrogen, measured content, pH value;
[0025] c. After the drug solution is qualified, it is filtered through a 0.2μm microporous membrane, and after filling in a 100-grade environment, it is sterilized once by circulatin...
Embodiment 3
[0031] Comparison of the injection prepared in the examples of the present invention with the injection prepared according to the prior art.
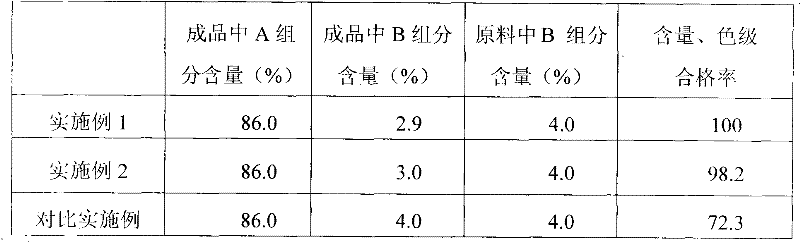
[0032] For the lincomycin in the injections prepared in Example 1, Example 2 and Comparative Example, the contents of component A and component B were measured by high-performance liquid chromatography, and the test results are shown in Table 1 as follows. The content and color grade of the prepared injection were stored under the same conditions for 3 years, and tested according to the provisions of the Pharmacopoeia. The qualified rate of the test is shown in Table 1.
[0033] Table 1:
[0034]
[0035] The test results show that the method of the invention can reduce the content of lincomycin B in the finished product from 4.0% to about 3.0%, which is more than 1% lower than the original process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com