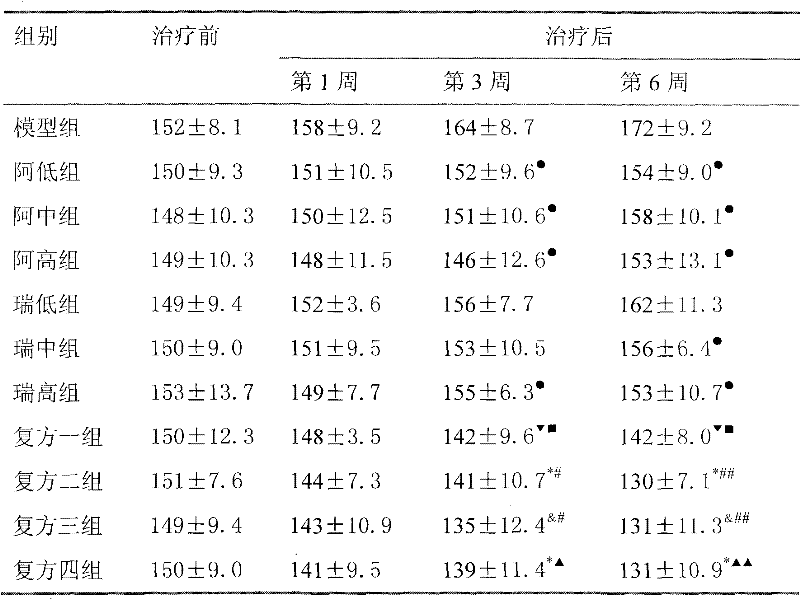
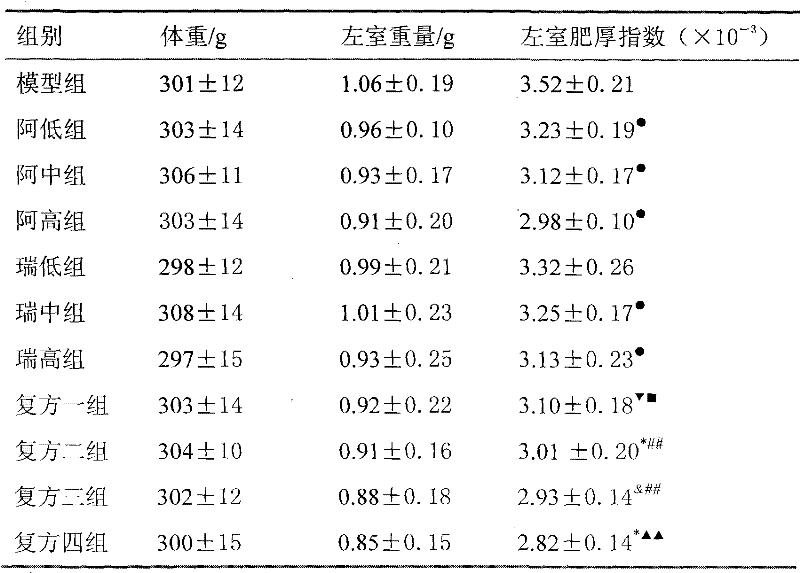
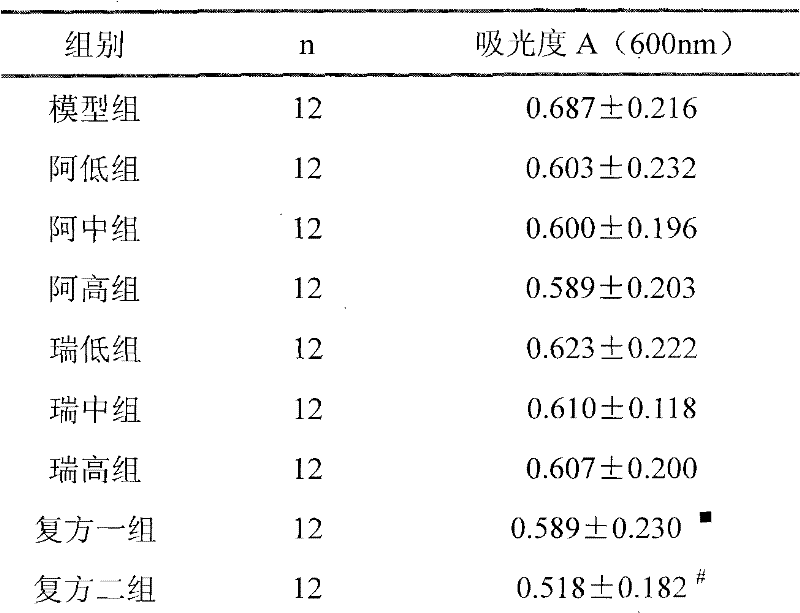
Medicine composition used for treating hypertension
A composition and high blood pressure technology, applied in the direction of drug combination, pharmaceutical formula, cardiovascular system diseases, etc., can solve the problem of rosuvastatin calcium, and the drug composition of aliskiren and rosuvastatin calcium, which is not disclosed Pharmacodynamic tests and their results and other issues, to achieve good preventive effect, kidney protection, and good synergistic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 Pharmaceutical composition common tablet of the present invention
[0022] Rosuvastatin Calcium 1g
[0023] Aliskiren 120g
[0024] Starch 140g
[0025] Dextrin 120g
[0026] 50% ethanol appropriate amount
[0027] Magnesium Stearate 1.0g
[0028] Preparation process: Weigh the prescribed amount of rosuvastatin calcium, aliskiren, starch, and dextrin and mix evenly. In addition, add an appropriate amount of 50% ethanol to the mixed powder, mix evenly, make soft material, pass through a 18-mesh nylon sieve to make wet granules, and dry at about 60°C. The moisture content of the dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
Embodiment 2
[0029] Embodiment 2 Pharmaceutical composition capsule of the present invention
[0030] Rosuvastatin Calcium 1g
[0031] Aliskiren 3.75g
[0032] Microcrystalline Cellulose 300g
[0033] Micronized silica gel 12g
[0034] Preparation process: Grind rosuvastatin calcium, aliskiren, microcrystalline cellulose, and micropowder silica gel through a 100-mesh sieve, mix well, and directly fill capsules.
Embodiment 3
[0035] Embodiment 3 Pharmaceutical composition bilayer tablet of the present invention
[0036] Rosuvastatin Calcium 10g
[0037] Mannitol 10g
[0038] Lactose 40g
[0039] Microcrystalline Cellulose 20g
[0040] 6% PVP in 95% ethanol solution 120g
[0042] Preparation process a: Rosuvastatin calcium is passed through a 100-mesh sieve, mannitol, lactose, and microcrystalline cellulose are passed through a 80-mesh sieve, and the prescription amount of rosuvastatin calcium is weighed and mixed with mannitol, lactose, and microcrystalline cellulose evenly , adding 6% PVP in 95% ethanol solution in an appropriate amount to granulate, drying at 60°C, sieving the dry granules with a 16-mesh sieve, and adding a prescribed amount of magnesium stearate to the dry granules.
[0043] Aliskiren 37.5g
[0044] Pregelatinized starch 50g
[0045] Mannitol 50g
[0046] 6% PVP in 95% ethanol solution 100g
[0047] Micronized silica gel 5g
[0048] Prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com