Cobaltosic oxide preparation method
A technology of tricobalt tetroxide and cobalt powder, which is applied in the direction of cobalt oxide/cobalt hydroxide, etc., which can solve the problems of difficult control of product morphology, complex preparation process, and wide pore size distribution, and achieve easy control, simple preparation process, and uniform particle size and shape effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh 1 mmol of cobalt chloride and 1 mmol of oxalic acid and dissolve in 10 ml of nitrogen, nitrogen dimethylacetamide (DMA) to obtain a blue transparent solution. 8 milliliters of deionized water was added thereto, and stirred to obtain a hydrated cobalt oxalate precipitate. The precipitate was washed with deionized water and dried in air to obtain a hydrated cobalt oxalate nanorod structure with good crystal shape.
[0022] The dry powder obtained in the above steps is placed in a muffle furnace for heat treatment at 350° C. for 0.5-3 hours, and then cooled to room temperature to obtain a mesoporous cobalt trioxide nanorod structure.
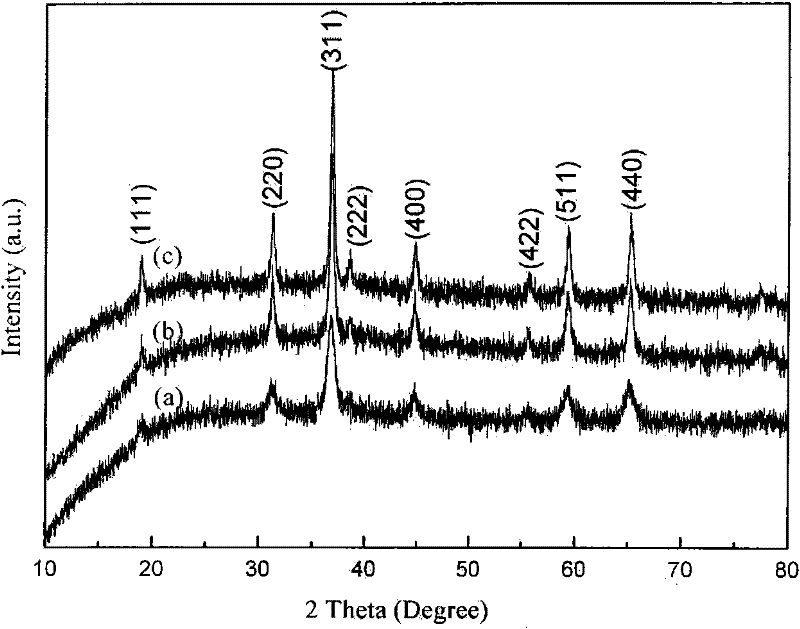
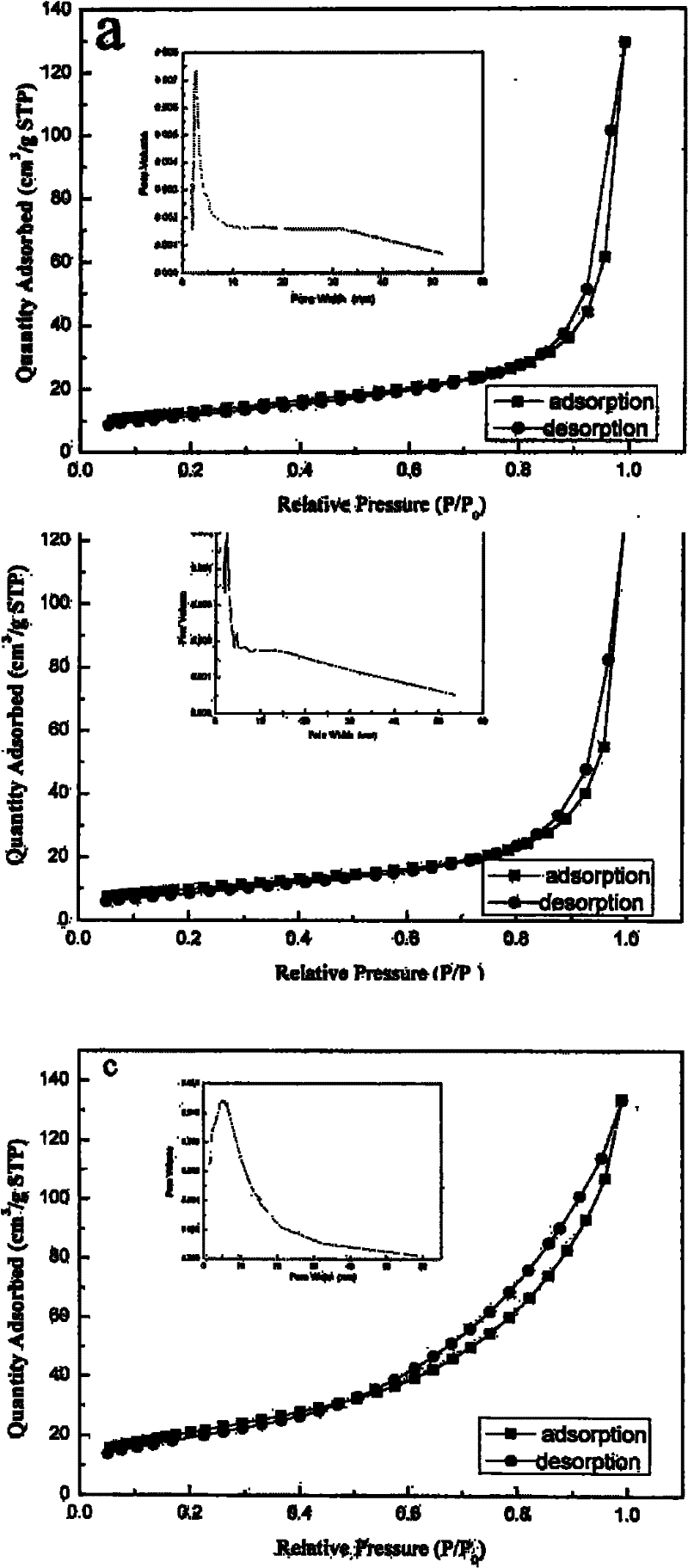
[0023] Utilize the X'Pert PRO type X-ray powder diffractometer (Cu Kα radiation of PANalytical Company, λ=1.54056 ), to characterize the phase structure of the product, and its test structure is as figure 1 As shown, the product is a pure phase spinel structure of cobalt tetraoxide with good crystallinity. Nitrogen adsorption-desor...
Embodiment 2
[0025] Weigh 1 mmol of cobalt chloride and 1 mmol of oxalic acid and dissolve in 10 ml of nitrogen, nitrogen dimethylacetamide (DMA) to obtain a blue transparent solution. Then it was added to 8 ml of deionized water and stirred to obtain a precipitate of hydrated cobalt oxalate. The precipitate was washed with deionized water and dried in air to obtain a hydrated cobalt oxalate nanowire structure with good crystal shape.
[0026] The dry powder obtained in the above steps is placed in a muffle furnace for heat treatment at 350° C. for 0.5-3 hours, and then cooled to room temperature to obtain a mesoporous cobalt trioxide nanowire structure.
Embodiment 3
[0028] Weigh 1 mmol of cobalt chloride and 1 mmol of oxalic acid and dissolve in 10 ml of dimethyl sulfoxide (DMSO) to obtain a blue transparent solution. Then add 8 milliliters of deionized water therein, stir to obtain hydrated cobalt oxalate precipitate. The precipitate was washed with deionized water and dried in air to obtain a layered wrinkled structure of hydrated cobalt oxalate with good crystal shape.
[0029] The dry powder obtained in the above steps is placed in a muffle furnace for heat treatment at 350° C. for 0.5-3 hours, and then cooled to room temperature to obtain a mesoporous cobalt trioxide nano-layered wrinkled structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com