Calcium titanate luminescent material and preparation method thereof
A luminescent material, calcium titanate technology, applied in luminescent materials, chemical instruments and methods, etc., can solve problems such as low luminous efficiency, reduced luminous efficiency, increased non-radiative transition probability, etc., and achieves simple preparation process, enhanced luminescence, and equipment less demanding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
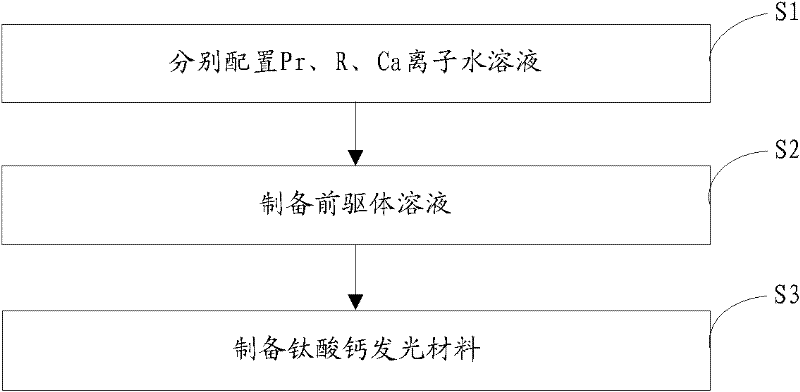
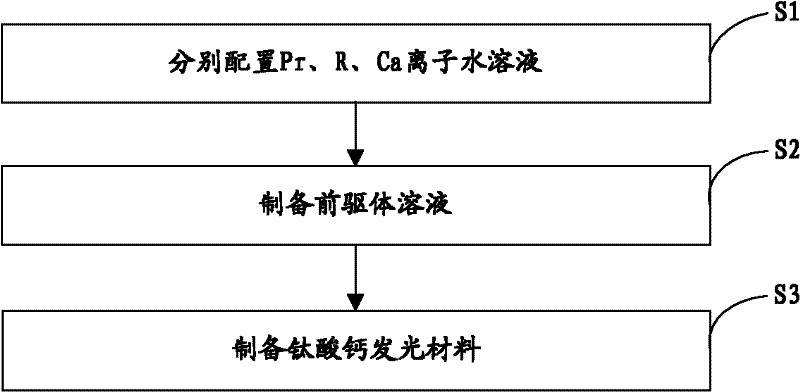
[0022] The invention provides a preparation method of calcium titanate luminescent material, such as figure 1 As shown, the preparation process is as follows:
[0023] Step S1, according to the general chemical formula Ca 1-x Ti 1-y o 3 : The stoichiometric ratio of each element in xPr, yR provides the source compound of Pr, the source compound of R and the source compound of Ca, and then configures the molar concentration of Pr ion to be 0.05~0.4mol / L and the molar concentration of R ion to be 0.05 respectively ~0.5mol / L and an aqueous solution with a Ca ion molar concentration of 1~2mol / L; wherein, 0.001≤x≤0.01, 0.005≤y≤0.03, R is at least one of Al or Ga;
[0024] Step S2, adding 3.52 mL of tetrabutyl titanate to an ethanol solution of citric acid (wherein, the molar concentration of citric acid is 7×10 -7 ~2×10 -6 mol / L), stirred for 30 minutes, then added 0.2-0.6 mL of the aqueous solution containing Pr ions and 0.2-2 mL of the aqueous solution containing R ions prep...
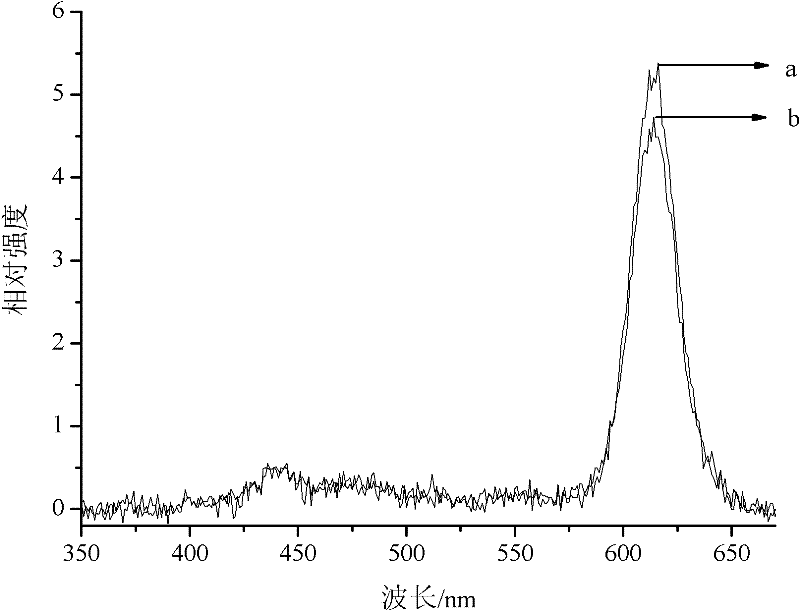
Embodiment 1
[0031] Weigh 2.1751g of praseodymium nitrate to make a 100mL 0.05mol / L solution; 1.8757g of aluminum nitrate to make a 100mL 0.05mol / L solution; 23.615g of calcium nitrate to make a 100mL 1mol / L solution. Weigh 0.4247g silver nitrate, dissolve in deionized water, and make 100mL 1×10 -2 mol / L solution.
[0032] Weigh 0.04mol or 7.6848g of citric acid and place it in a dry beaker, add 20mL of absolute ethanol, continue to stir for 30min after the citric acid is dissolved, slowly add 3.52mL of tetrabutyl titanate, gradually form a light yellow liquid, slowly drop in nitric acid Praseodymium 0.2mL and aluminum nitrate 0.2mL, after stirring evenly, slowly add calcium nitrate solution 9.99mL and silver nitrate solution 0.1mL to prevent hydrolysis of tetrabutyl titanate, continue stirring for 5h, heat up to 50°C, evaporate to dryness, from 50 ℃ gradually increased to 150 ℃ and dried in an oven to obtain tan precursor powder. The precursor was placed in a muffle furnace, heated to 50...
Embodiment 2
[0034] Weigh 8.7002g of praseodymium nitrate to make a 100mL 0.2mol / L solution; 11.2539g of aluminum nitrate to make a 100mL 0.3mol / L solution; 35.4225g of calcium nitrate to make a 100mL 1.5mol / L solution. Weigh 0.0412g of chloroauric acid, dissolve in deionized water, and make 100mL 1×10 -3 mol / L solution.
[0035] Weigh 0.04mol or 7.6848g of citric acid and put it in a dry beaker, add 40mL of absolute ethanol, continue to stir for 30min after the citric acid is dissolved, slowly add 3.52mL of tetrabutyl titanate, gradually form a light yellow liquid, slowly drop in nitric acid 0.5mL of praseodymium and 1mL of aluminum nitrate, after stirring evenly, slowly add 6.6mL of calcium nitrate solution and 0.1mL of chloroauric acid solution to prevent hydrolysis of tetrabutyl titanate, continue stirring for 1h, raise the temperature to 70°C, evaporate to dryness, ℃ gradually increased to 150 ℃ and dried in an oven to obtain tan precursor powder. The precursor was placed in a muffle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com