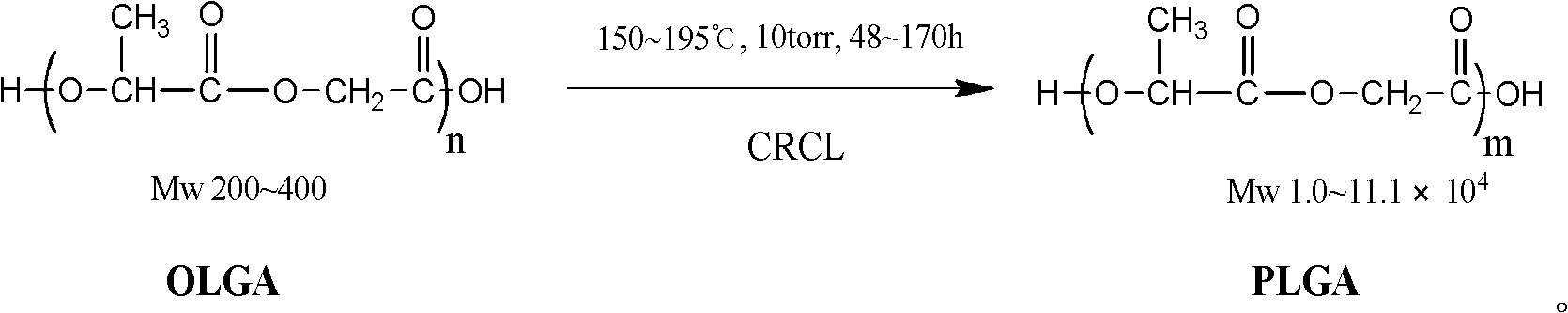
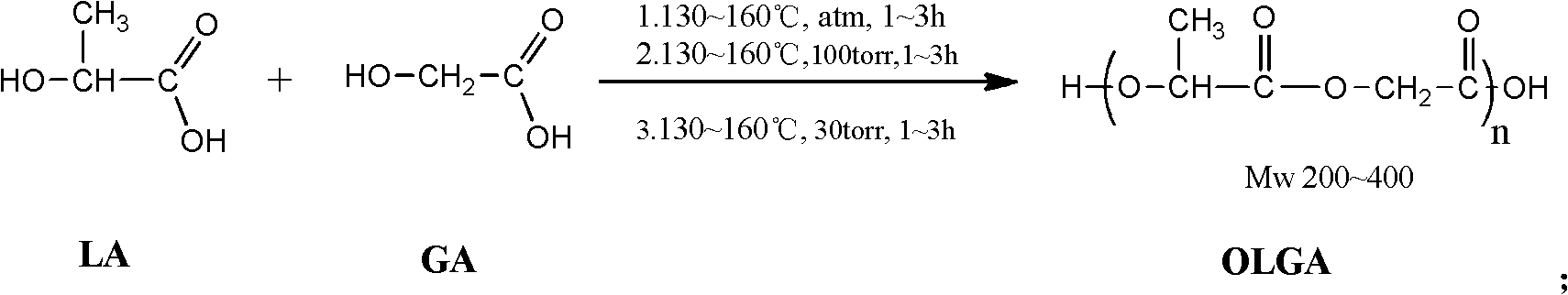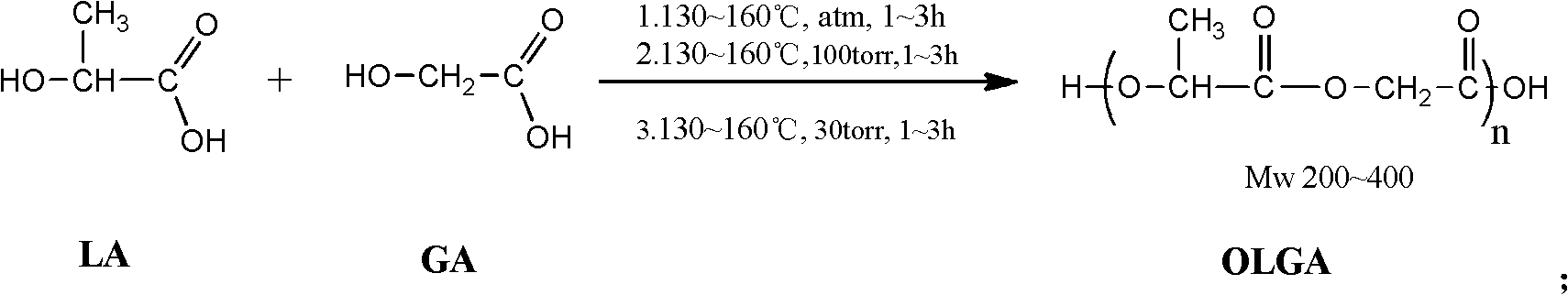Process method for catalytic synthesis of poly lactic acid-glycolic acid by using bionic organic guanidinium
A technology for catalyzing lactic acid and glycolic acid with guanidine chloride creatinine, which is applied in the field of medical biodegradable materials, can solve problems such as potential safety hazards, and achieve the effects of simple process operation, low cost of raw materials, excellent biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 - the synthesis of oligomeric lactic acid-glycolic acid
[0026] Put 45g of industrial-grade lactic acid aqueous solution LA with a mass content of 85% and 34g of glycolic acid GA with a mass content of 95% in the reaction kettle, repeat vacuuming-after three times of argon filling, heat under argon atmosphere and normal pressure To 130°C, dehydration reaction for 3 hours. Then the reactor was depressurized to 100 Torr and reacted at 130° C. for 3 hours. Finally, the reactor was depressurized to 30 Torr and reacted at 130° C. for 3 hours to obtain oligomeric lactic acid-glycolic acid OLGA with a yield of 98.6% and a weight average molecular weight of 220.
Embodiment 2
[0027] Embodiment 2 - the synthesis of oligomeric lactic acid-glycolic acid
[0028] Put 45g of industrial-grade lactic acid aqueous solution LA with a mass content of 85%, and 44.1g of glycolic acid GA with a mass content of 95% in the reactor, and repeat vacuuming—after three times of argon filling, heat under argon atmosphere and normal pressure To 160°C, dehydration reaction for 1 hour. Then the reactor was depressurized to 100 Torr and reacted at 160° C. for 1 hour. Finally, the reactor was decompressed to 30 Torr and reacted at 160° C. for 1 hour to obtain oligomeric lactic acid-glycolic acid OLGA with a yield of 98.2% and a weight average molecular weight of 320.
Embodiment 3
[0029] Embodiment 3 - the synthesis of oligomeric lactic acid-glycolic acid
[0030] Put 45g of industrial-grade lactic acid aqueous solution LA with a mass content of 85% and 14.6g of glycolic acid GA with a mass content of 95% in the reaction kettle and repeat vacuuming—after three times of argon filling, heat under argon atmosphere and normal pressure To 145°C, dehydration reaction for 2 hours. Then the reactor was depressurized to 100 Torr and reacted at 145° C. for 2 hours. Finally, the reactor was decompressed to 30 Torr and reacted at 145° C. for 2 hours to obtain oligomeric lactic acid-glycolic acid OLGA with a yield of 98.4% and a weight average molecular weight of 390.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com