Preparation methods of lanthanum-doped cobalt nickel ferrite gas-sensitive powder and gas sensor
A technology of cobalt-nickel ferrite and gas sensor, applied in the direction of material resistance, can solve problems such as low sensitivity, and achieve the effects of high sensitivity, good selectivity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 1.8181g Fe(NO 3 ) 3 9H 2 O, 0.5821g Co(NO 3 ) 2 ·6H 2 O, 0.1454g Ni(NO 3 ) 2 ·6H 2 O, 0.2165g La(NO 3 ) 3 ·6H 2 O is added to a three-necked flask, and then 50ml of analytically pure ethylene glycol solution is added, and after fully stirring, Fe in the solution 3+ The molar concentration is 0.09mol / L; then add 3.6g of anhydrous sodium acetate and mechanically stir for 25min at a speed of 500rpm. 12h. After the reaction was completed, the product was washed with distilled water and absolute ethanol, and centrifuged three times, and then the product was dried at 80°C for 12 hours to obtain Co 0.8 Ni 0.2 La 0.2 Fe 1.8 o 4 Nano powder.
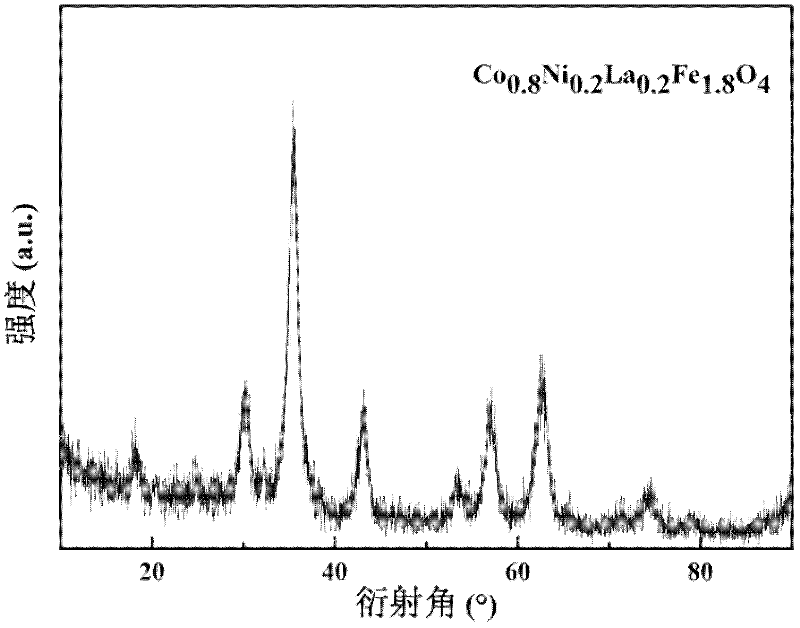
[0034] figure 2 It is the X-ray diffraction pattern of the powder synthesized in this embodiment, and the diffraction peaks in the figure show that the nanopowder is Co 0.8 Ni 0.2 La 0.2 Fe 1.8 o 4 , the Scherrer formula calculation shows that the grain size of the synthetic powder is about 5.4nm; image 3 It...
Embodiment 2
[0038] Weigh 1.9191g Fe(NO 3 ) 3 9H 2 O, 0.5821g Co(NO 3 ) 2 ·6H 2 O, 0.1454g Ni(NO 3 ) 2 ·6H 2 O, 0.1083g La(NO 3 ) 3 ·6H 2 0, add a three-necked flask, then add 55ml of analytically pure ethylene glycol solution, after fully stirring, Fe in the solution 3+ The molar concentration is 0.085mol / L; then add 4.42g of anhydrous sodium acetate and mechanically stir for 40min at a speed of 300 rpm. 8h; after the reaction was completed, the product was washed with distilled water and absolute ethanol, and centrifuged for 4 times, and then the product was dried at 60°C for 18h to obtain Co 0.8 Ni 0.2 La 0.1 Fe 1.9 o 4 Nano powder.
[0039] X-ray diffraction analysis shows that the nanopowder is Co 0.8 Ni 0.2 La 0.1 Fe 1.9 o 4 ; It can be seen from the scanning electron microscope photo of the synthetic powder that the Co in the powder 0.8 Ni 0.2 La 0.1 Fe 1.9 o 4 The particle size is about 100nm.
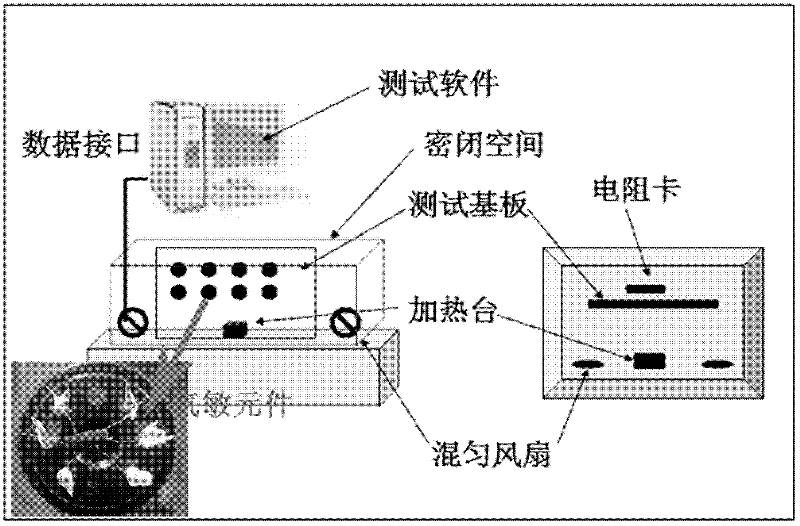
[0040] Weigh 0.08g of Co 0.8 Ni 0.2 La 0.1 Fe 1.9 o 4 T...
Embodiment 3
[0043] Weigh 1.5151g Fe(NO 3 ) 3 9H 2 O, 0.1455g Co(NO 3 ) 2 ·6H 2 O, 0.5816g Ni(NO 3 ) 2 ·6H 2 O, 0.5413g La(NO 3 ) 3 ·6H 2 0, add a three-necked flask, then add 50ml of analytically pure ethylene glycol solution, after fully stirring, Fe in the solution 3+ The molar concentration is 0.094mol / L; then add 4.62g of anhydrous sodium acetate, and mechanically stir for 35min at a speed of 400 rpm. 14h. After the reaction was completed, the product was washed with distilled water and absolute ethanol, and centrifuged for 5 times, and then the product was dried at 50°C for 22h to obtain Co 0.2 Ni 0.8 La 0.5 Fe 1.5 o 4 Nano powder.
[0044] X-ray diffraction analysis shows that the nanopowder is Co 0.8 Ni 0.2 La 0.1 Fe 1.9 o 4 ; It can be seen from the scanning electron microscope photo of the synthetic powder that the Co in the powder 0.2 Ni 0.8 La 0.5 Fe 1.5 o 4 The particle size is about 100nm.
[0045] Weigh 0.09g of Co 0.2 Ni 0.8 La 0.5 Fe 1.5 o ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com