Zinc chelate-containing compound vitamin oral nano-emulsion and preparation method for same
A technology for compound vitamins and milk preparations, applied in the directions of medical preparations, drug combinations, and pharmaceutical formulations containing active ingredients, can solve problems such as precipitation, affecting the normal efficacy of products, precipitation, etc., achieving simple process, enhanced antioxidant effect, The effect of promoting absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A compound vitamin oral nanoemulsion preparation containing chelated zinc, the components per liter are as follows:
[0031] Zinc Gluconate 75g, Lysine Hydrochloride 60g, Vitamin A 5g, Vitamin D 3 0.7g, vitamin E 20g, sodium selenite 0.2g, Tween-80 250g, propylene glycol 120g, ethylparaben 0.2g, 2,6-di-tert-butyl-4-methylphenol (BHT) 0.2g , water to 1L.
[0032] The preparation method of the above-mentioned compound vitamin oral nanoemulsion preparation containing chelated zinc comprises the steps:
[0033] (1) Weigh zinc gluconate and lysine hydrochloride in water in proportion, fully stir and dissolve at 40°C, then add sodium selenite, after the solution is clarified, pass through a 5 μm filter membrane, and set aside;
[0034] (2) Weigh VA oil, VD3 oil, and VE oil in proportion and pour them into a dry reaction tank, add preservatives and antioxidants, stir until they are completely dissolved, add emulsifiers and co-emulsifiers, stir until they are evenly clarifie...
Embodiment 2
[0072] A compound vitamin oral nanoemulsion preparation containing chelated zinc, the components per liter are as follows:
[0073] Zinc gluconate 76g, methionine 55g, vitamin A 6g, vitamin D 3 0.6g, vitamin E 15g, sodium selenite 0.3g, Tween-80 280g, polyethylene glycol 100g, ethylparaben 0.2g, BHT 0.2g, dilute water to 1L.
[0074] The preparation method is the same as in Example 1.
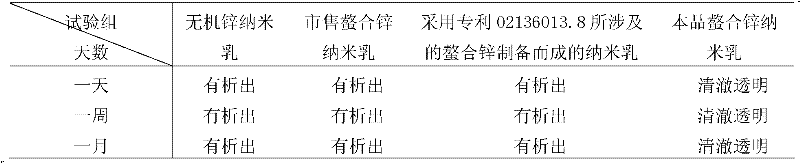
[0075] Reserved sample observation test
[0076] Take part of the nanoemulsion prepared in Example 2, divide it into several glass bottles, seal it and place it under the conditions of 0°C, 25°C, and 40°C for 180 days, and take samples for observation every 10 days. The results showed that the nanoemulsion maintained a clear and transparent appearance under the above five temperature conditions, without precipitation, precipitation, opalescence and other phenomena. Observation under a transmission electron microscope shows that the droplets of the nanoemulsion are spherical, uniformly distr...
Embodiment 3
[0080] A compound vitamin oral nanoemulsion preparation containing chelated zinc, the components per liter are as follows:
[0081] Zinc gluconate 78g, methionine 68g, vitamin A 10g, vitamin D 3 0.5g, vitamin E 23g, sodium selenite 0.2g, polyoxyethylene castor oil EL-35 300g, polyethylene glycol 150g, ethylparaben 0.4g, BHT 0.2g, dilute water to 1L.
[0082] The preparation method is the same as in Example 1.
[0083] Reserved sample observation test
[0084] Take part of the nanoemulsion prepared in Example 3, divide it into several glass bottles, seal it and place it under the conditions of 0°C, 25°C, and 40°C for 180 days, and take samples for observation every 10 days. The results show that the nanoemulsion maintains a clear and transparent appearance under the above three temperature conditions, without precipitation, precipitation, opalescence and other phenomena. Observation under a transmission electron microscope shows that the droplets of the nanoemulsion are sph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com