Tiopronin sterile powder and preparation and preparation method thereof
A technology for tiopronin and sterile powder injection, which is applied in the field of sterile tiopronin powder and its preparation and preparation, can solve the problems of decomposition of tiopronin, easy generation of impurities, poor stability, etc., and achieves high yield and purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
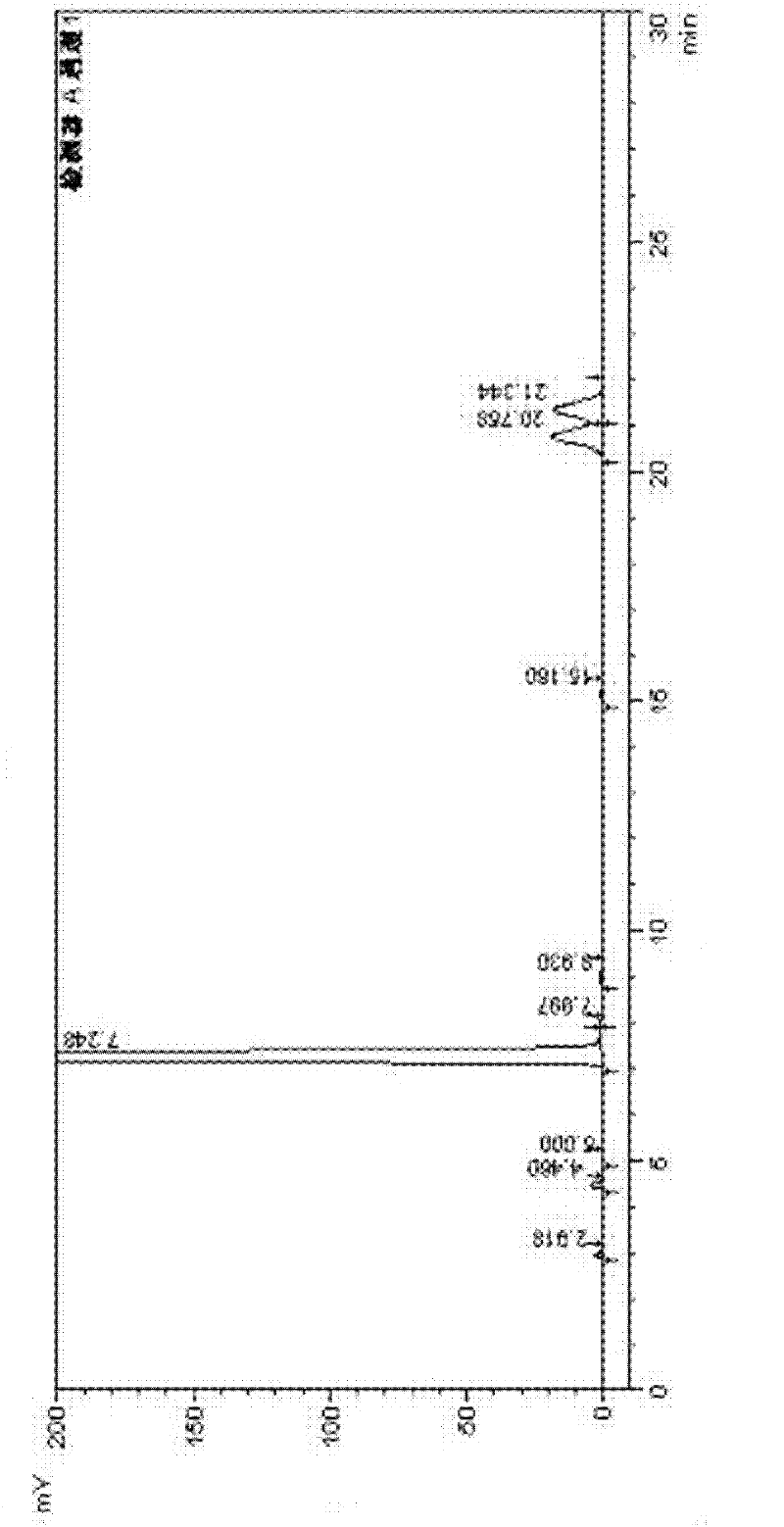
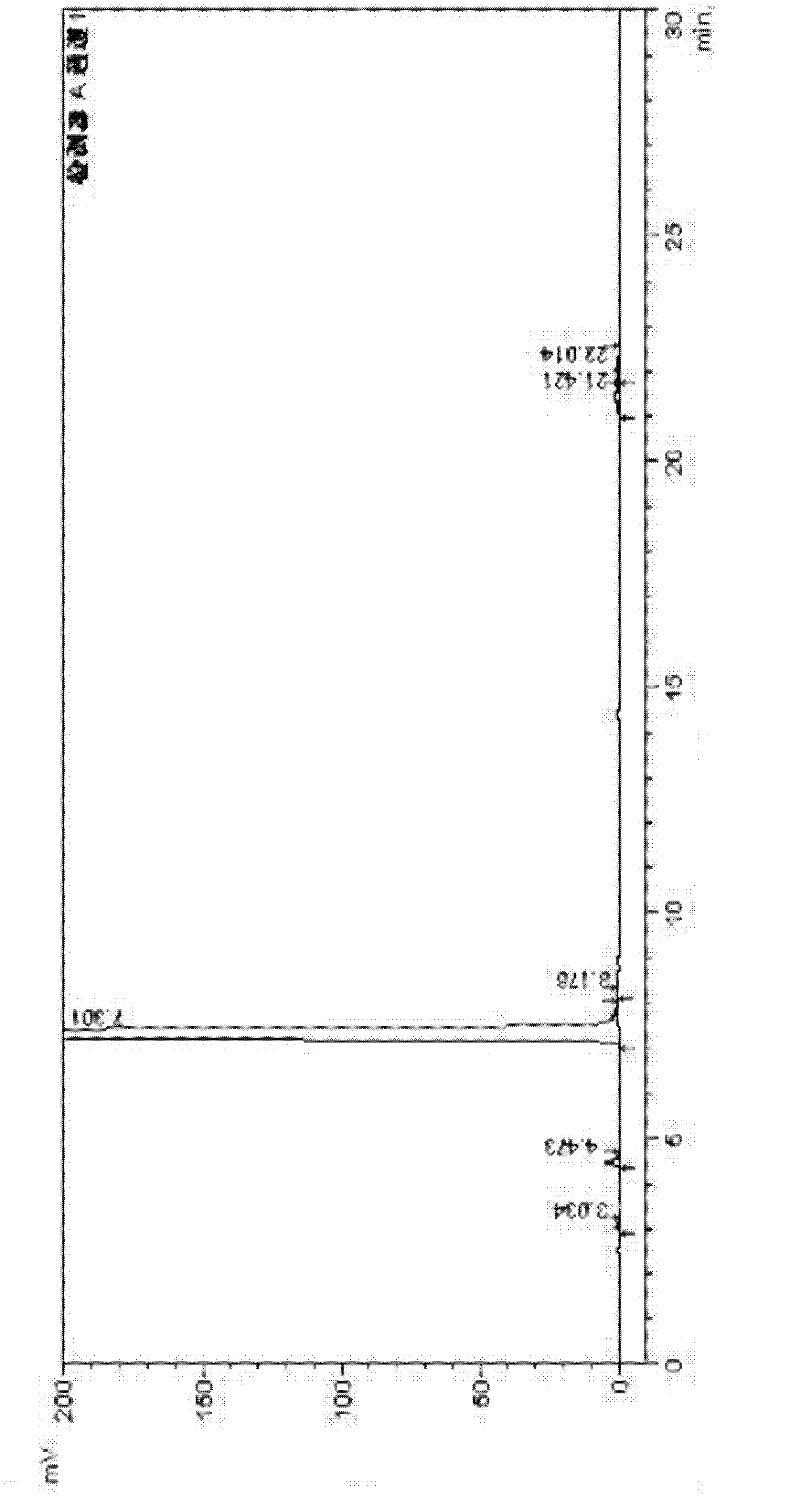
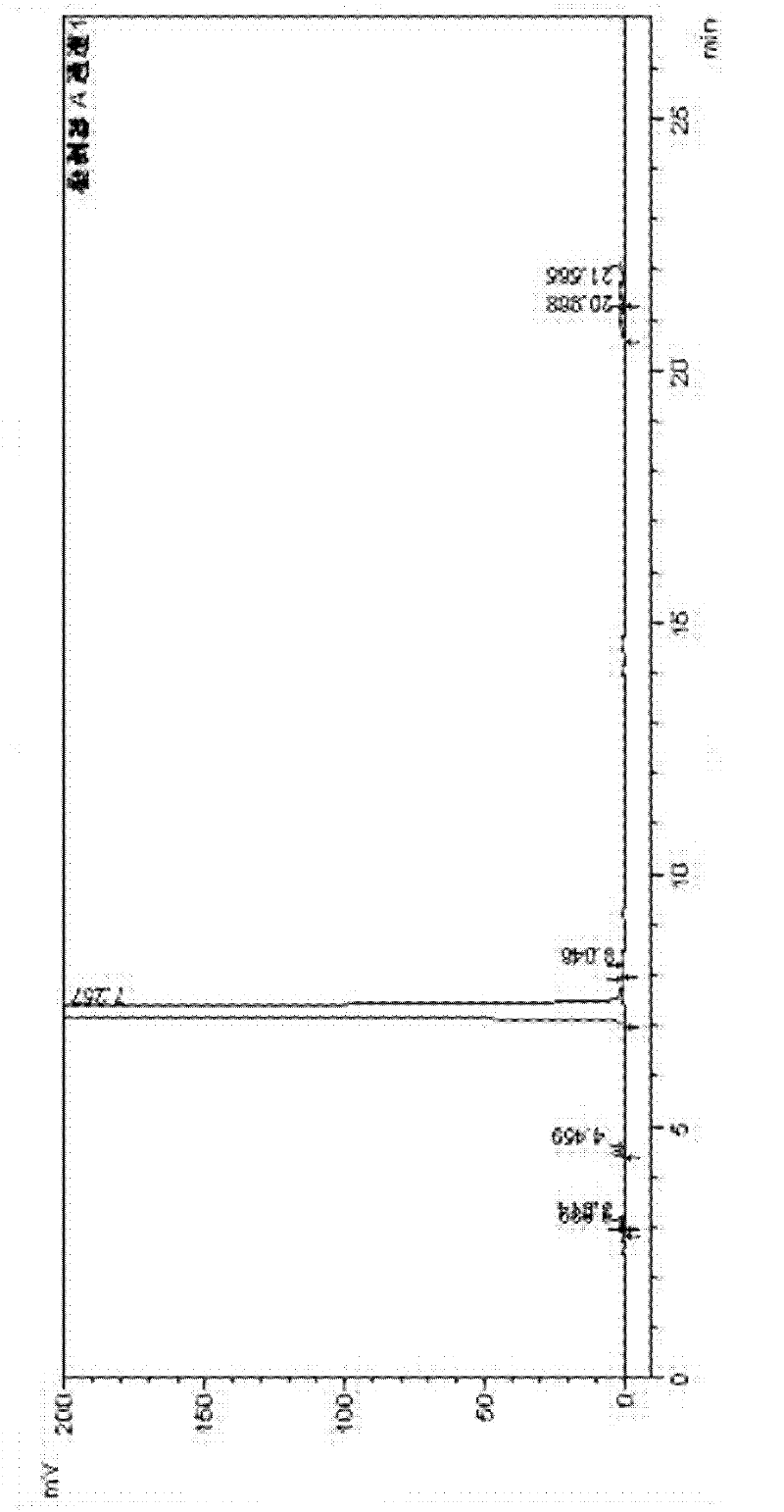
Image
Examples
preparation example Construction
[0031] A preparation method of tiopronin aseptic powder, comprising:
[0032] Step 1: Take tiopronin raw material, add 4 to 10 times ethyl formate in g / mL to dissolve, heat to reflux, add 0.1% to 0.5% injection in weight / volume percentage in g / mL Adsorb with activated carbon and filter;
[0033] Step 2: Take the filtrate obtained in Step 1, add 5v / v% to 30v / v% of ethyl formate ether or methyl tert-butyl ether, heat and stir to dissolve, and filter it with a 0.22μm microporous membrane below 0.1Mpa;
[0034] Step 3: Freeze the filtrate obtained in step 2 to -5-5°C for crystallization, collect the obtained crystals, filter, dry under reduced pressure at 15-30°C, and pulverize.
Embodiment 1
[0035] In order to further understand the present invention, the present invention will be described in detail below in conjunction with examples. Embodiment 1: the preparation of tiopronin sterile powder injection
[0036] (1) Take by weighing 500g tiopronin raw material, purity is 95.3%, add in the reactor, add 6 times of amount, i.e. ethyl formate of 3L, heat reflux;
[0037] (2) After tiopronin is dissolved, add 9g of activated carbon for injection, and filter under reflux for 30 minutes;
[0038] (3) After filtering, add 300mL methyl tert-butyl ether to the filtrate, heat and stir to dissolve;
[0039] (4) After dissolving, below 0.1Mpa, use a 0.22μm microporous membrane to filter under positive pressure;
[0040] (5) After filtering, freeze the filtrate to 0°C, and crystallize at low temperature for 2 hours;
[0041] (6) After the crystallization is completed, filter, and vacuum-dry the obtained crystals at 15° C. for 4 hours;
[0042](7) After drying, the crystals w...
Embodiment 2
[0044] Embodiment 2: the preparation of tiopronin sterile powder injection
[0045] (1) Take by weighing 500g tiopronin raw material, purity is 95.3%, add in the reactor, add 10 times of amount, i.e. ethyl formate of 5L, heat to reflux;
[0046] (2) After tiopronin is dissolved, add 25g of active carbon for injection, and filter under reflux for 30 minutes;
[0047] (3) After filtering, add 1000mL methyl tert-butyl ether to the filtrate, heat and stir to dissolve;
[0048] (4) After dissolving, below 0.1Mpa, use a 0.22μm microporous membrane to filter under positive pressure;
[0049] (5) After filtering, freeze the filtrate to 5°C, and crystallize at low temperature for 2 hours;
[0050] (6) After the crystallization is completed, filter, and vacuum-dry the obtained crystals at 30° C. for 3 hours;
[0051] (7) After drying, the crystals were pulverized to obtain 456.5 g of white tiopronin sterile powder, with a yield of 91.3%. 99.5% purity;
[0052] (8) The sterile powde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com