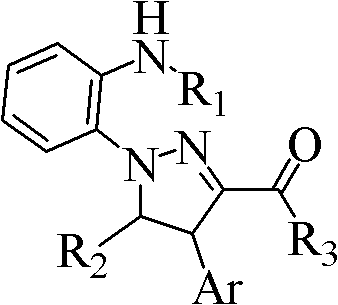
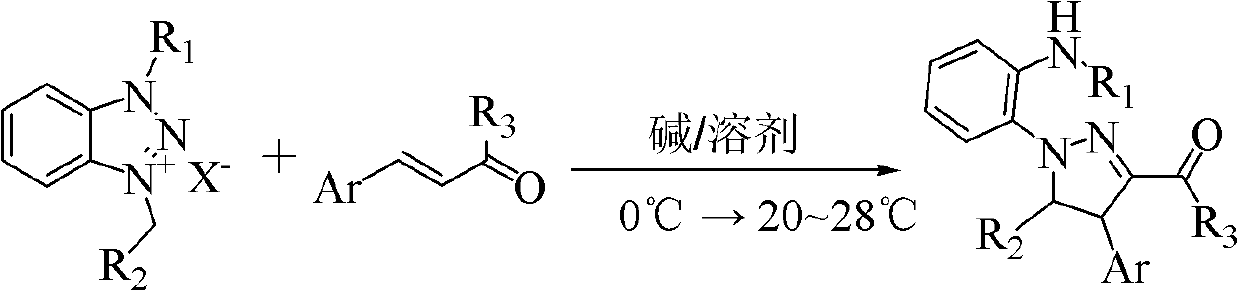
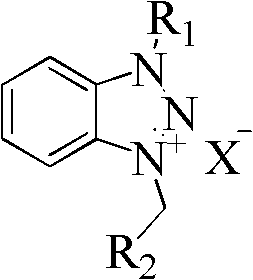
Poly-substituted pyrazoline compound and preparation method thereof
A pyrazoline and multi-substitution technology, which is applied in the field of multi-substituted pyrazoline compounds and their preparation, can solve problems such as difficulty in obtaining multi-substituted pyrazoline compounds, and achieve the effects of easy-to-obtain raw materials, mild reaction conditions, and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Synthesis of 1-[2-(methylamino)phenyl]-4,5-diphenyl-4,5-dihydro-1H-pyrazole-3-carbaldehyde
[0035] The reaction formula is:
[0036]
[0037] At room temperature, add 0.218g (1.65mmol) cinnamaldehyde, 0.456g (1.5mmol) 1-methyl-3-benzylbenzotriazole bromide, 10mL THF, 2mL DMSO into a 25mL round bottom flask and stir to dissolve. Cool down to 0°C, add 0.168g (1.5mmol) potassium tert-butoxide in one go, follow up with thin-layer chromatography (developing solvent: ethyl acetate:petroleum ether=1:15), after the reaction, concentrate and recover under reduced pressure THF, 15 mL of water was added to the residue, extracted with 3×20 mL of ethyl acetate, the combined organic phases were washed with 2×20 mL of saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to recover ethyl acetate. The residue was quickly separated by column chromatography (eluent: ethyl acetate:petroleum ether=1:15) to obtain 0.3437 g of an orange...
Embodiment 2
[0038] Example 2: 1-[2-(methylamino)phenyl]-5-phenyl-4-(4-methylphenyl)-4,5-dihydro-1H-pyrazole-3-carbaldehyde synthesis
[0039] The reaction formula is:
[0040]
[0041] The α,β-unsaturated aldehyde (ketone) used is p-methylcinnamaldehyde, and other raw materials, experimental methods, and reaction conditions are the same as in Example 1. The product obtained was an orange-red oily liquid with a yield of 57.5%. 1 H NMR (400MHz, CDCl 3 ), δ(ppm): 9.78(s, 1H), 7.30-7.19(m, 3H), 7.16-7.09(m, 4H), 7.07-7.03(m, 2H), 6.95(t, J=7.8Hz, 1H), 6.67(t, J=8.1Hz, 2H), 6.47(q, J=4.8Hz, 1H), 6.43(t, J=7.8Hz, 1H), 5.46(d, J=6.1Hz, 1H) , 4.30(d, J=6.1Hz, 1H), 2.92(d, J=5.0Hz, 3H), 2.30(s, 3H); 13 C NMR (100MHz, CDCl 3 ), δ (ppm): 184.1, 147.7, 141.5, 139.6, 137.6, 137.4, 130.1, 129.5, 128.5, 127.2, 126.8, 125.6, 125.5, 118.5, 116.0, 111.6, 78.3, 55.9, 30.6, IR (21.3; KBr) cm -1 3413, 3129, 2814, 2796, 1650, 1513, 1401, 1147, 743, 701; HRMS (EI): theoretical value [M] + (C 24 h ...
Embodiment 3
[0042] Example 3: 4-(4-methoxyphenyl)-1-[2-(methylamino)phenyl]-5-phenyl-4,5-dihydro-1H-pyrazole-3-carbaldehyde Synthesis
[0043] The reaction formula is:
[0044]
[0045] The α,β-unsaturated aldehyde (ketone) used is p-methoxycinnamaldehyde, and other raw materials, experimental methods, and reaction conditions are the same as in Example 1. The product obtained was an orange-red oily liquid with a yield of 60.4%. 1 H NMR (400MHz, CDCl 3 ), δ(ppm): 9.77(s, 1H), 7.28-7.18(m, 3H), 7.11(d, J=6.8Hz, 2H), 7.07(d, J=8.6Hz, 2H), 6.94(t , J=7.8Hz, 1H), 6.84(d, J=8.8Hz, 2H), 6.70-6.64(m, 2H), 6.48(q, J=4.6Hz, 1H), 6.42(t, J=7.6Hz , 1H), 5.44(d, J=6.1Hz, 1H), 4.28(d, J=6.1Hz, 1H), 3.70(s, 3H), 2.91(d, J=4.3Hz, 3H); 13 C NMR (100MHz, CDCl 3 ), δ (ppm): 184.2, 159.2, 147.8, 141.5, 139.6, 132.5, 129.5, 128.5, 128.4, 126.8, 125.6, 125.5, 118.4, 116.0, 114.7, 111.6, 78.3, 55.6, 55.4, 30.5; )cm -1 3428, 3129, 2788, 1648, 1510, 1401, 1148, 741, 699; HRMS (EI): theoretical value [M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com