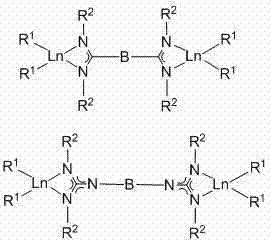
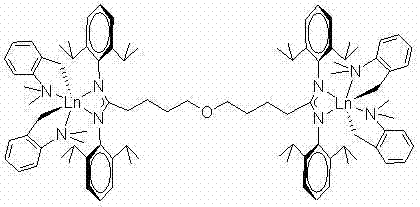
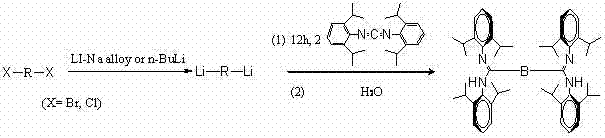Catalytic system for catalyzing polymerization of conjugated diolefins with bridging amidino-guanidyl dual-core rare-earth metals
A technology of rare earth metals and bridged amidines, which is applied in the field of guanidine dinuclear rare earth metal catalyst systems and bridged amidines, which can solve problems such as different properties, unknowns, and changes in polymerization selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 The synthesis of alkyl bridged ligands, the preparation process is shown in the figure below:
[0056]
[0057] Weigh 0.416 g (60 mmol) of lithium-sodium alloy, add 10 mL of ether, add 1.18 mL (10 mmol) of 1,4-dibromobutane dropwise at -10 °C, and continue the reaction for 24 hours after the addition is complete. Filter the reaction product with a sand core funnel to obtain a diethyl ether solution of 1,4-butyldilithium. Slowly add the ether solution of 1,4-butyldilithium into the solution of 5.801 g of carbodiimide in tetrahydrofuran under stirring, and then stir for 10 hours after the addition. After the reaction was complete, 1 mL of water was added to quench the reaction, and the product was separated through a column to obtain 3.70 g of the target product, the alkyl bridged amidinyl ligand, with a yield of 47%. 1 H NMR (CDCl 3 , 400 MHz, RT) : δ = 0.98 (d, 12H, CH(C H 3 ) 2 ), 1.15 (d, 12H, CH(C H 3 ) 2 ), 1.18 (d, 12H, CH(C H 3 ) 2 ), 1.28 ...
Embodiment 2
[0058] Example 2 Synthesis of oxygen-containing heteroatom alkyl bridging ligand, the preparation process is shown in the figure below:
[0059]
[0060] Weigh 0.2923 g Li-Na alloy in the glove box, place it in a 50 mL Schlenk bottle, add a stirring bar and 5 mL ether. Pipette 1.65 mL (8.95 mmol) of 4,4'-dichlorobutyl ether into the constant pressure dropping funnel under the condition of flowing N2, slowly drop it into the Schlenk bottle within 15 minutes, and stir at -15 °C React for 24 hours, and filter to obtain the corresponding dilithium salt ether solution. In the glove box, weigh 6.4894 g (17.9 mmol) of carbodiimide and dissolve it with tetrahydrofuran. Use a dropper to slowly add dilithium salt ether solution into the dissolved carbodiimide, and stir for 12 hours. After taking it out, quickly add water to quench and stir for 10 minutes. The product was separated by column, and finally 5.34 g of white powdery solid was obtained, with a yield of 64.5%. 1 H NMR (C...
Embodiment 3
[0061] Example 3 Synthesis of phenyl bridged ligand, the preparation process is shown in the figure below:
[0062]
[0063] At room temperature, slowly drop 2.4 mL of n-butyllithium solution (6 mmol, 2.5 M n-hexane solution) into a stirred 40 mL n-hexane solution containing 0.476 g (2 mmol) of 1,4-dibromobenzene. Keep the temperature at 80°C for 12 hours, and filter through a sand funnel to obtain phenyl dilithium salt (1,4-Li 2 C 6 h 4 ). Dissolve dilithium salt in 20 mL THF at room temperature, stir, slowly drop into 20 mL THF solution containing 1.45 g (4 mmol) carbodiimide, and react for 12 hours to obtain a light brown slurry-like solid. After the reaction is completed, excess distilled water is added to obtain a neutral amidine ligand. The product was extracted with ether and spin-dried to give a yellow solid. The solid was recrystallized from n-hexane at -30°C to obtain 0.48 g of light yellow crystals with a yield of 30%. 1 H NMR (400 MHz, CDCl 3 , RT): δ = 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com