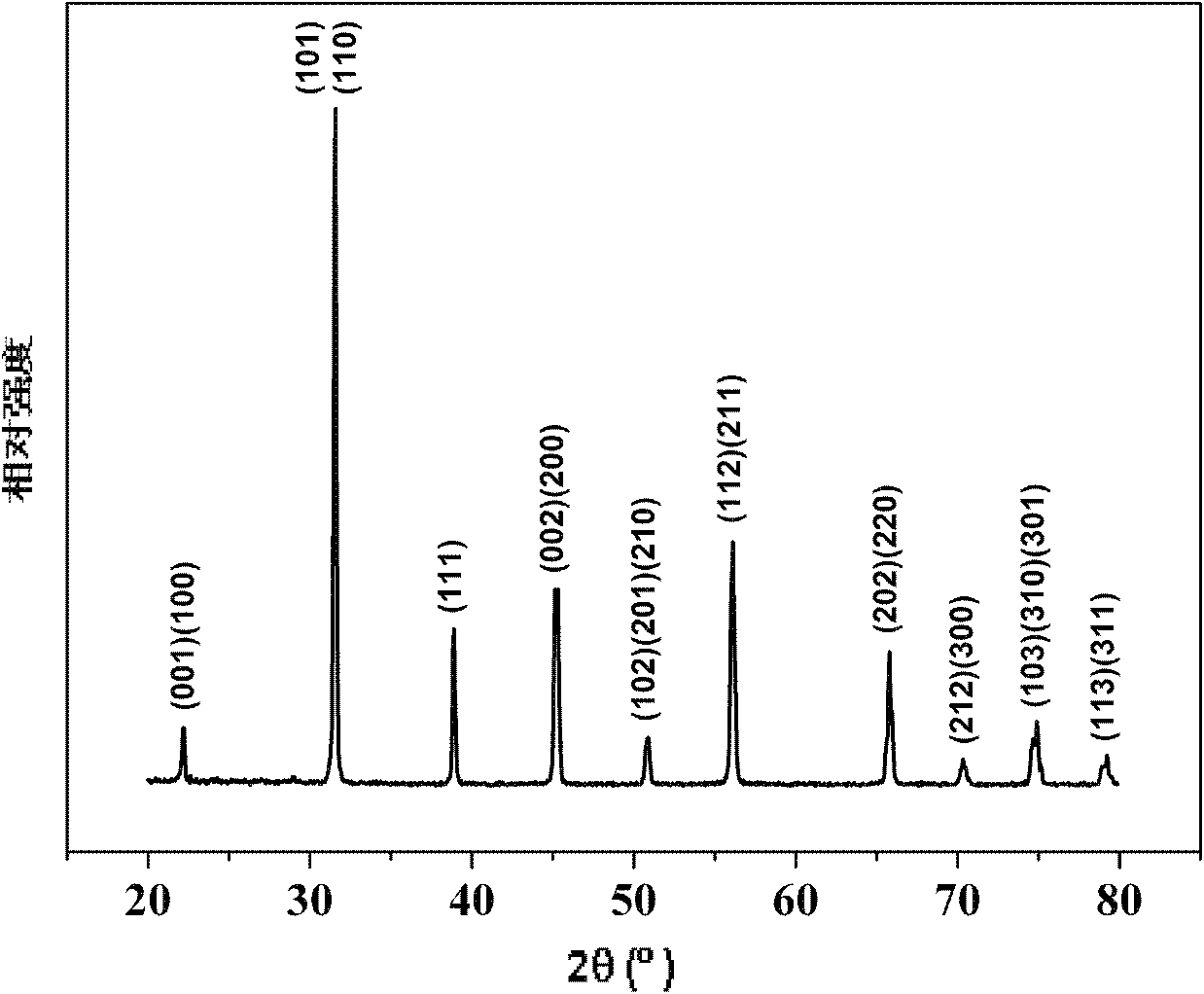
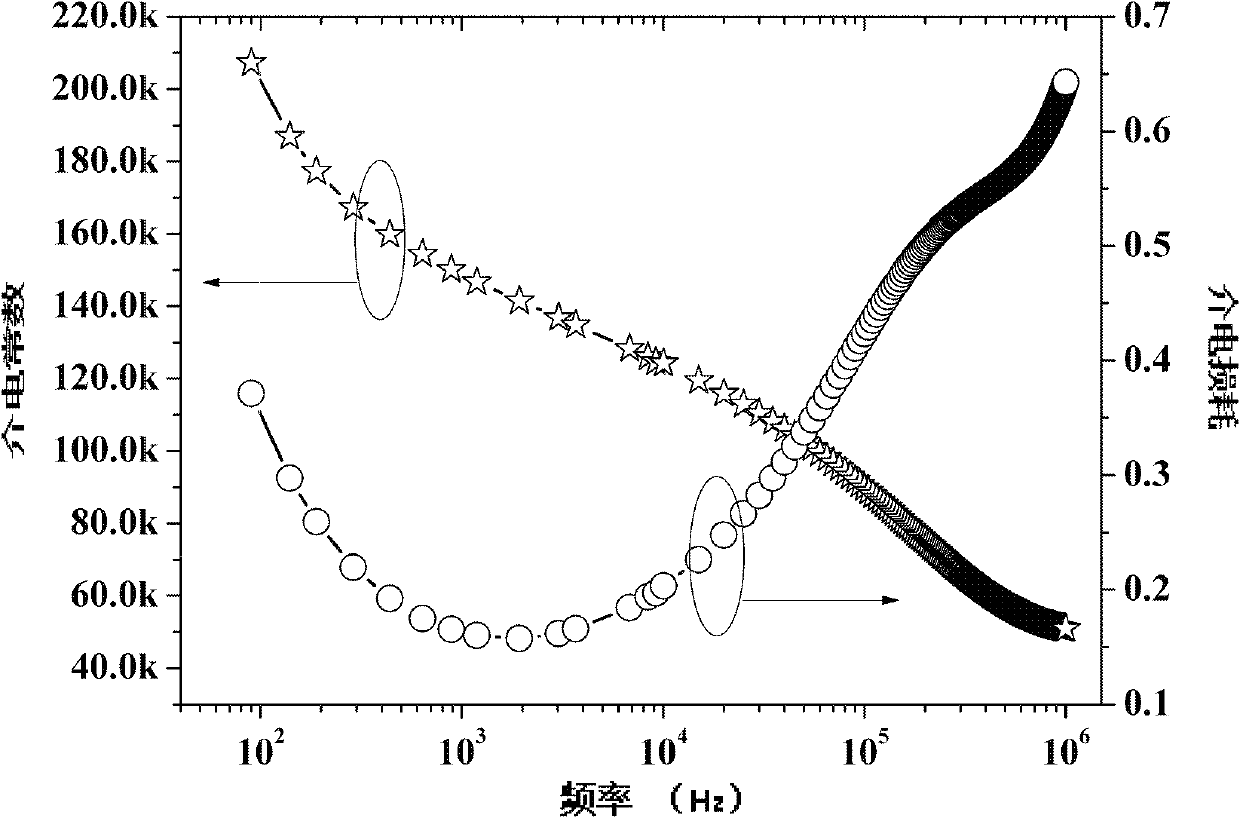
Method for preparing multi-component trace codoping zirconium barium strontium titanate-based micro powder
A technology of strontium barium zirconate titanate and co-doping, which is applied in the field of powder preparation by wet chemical method, can solve the problems that the uniformity of chemical components of materials is difficult to control, unsuitable, and difficult to distribute uniformly, so as to be beneficial to human health and environmental protection , the uniformity of the composition is improved, and the powder particle size distribution is narrow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Ba 0.96 Sr 0.04 Ti 0.955 Y 0.005 Zr 0.04 o 2.9975 Preparation of micropowder
[0038] (1) Preparation of a solution containing Ba, Sr ions
[0039] At room temperature and normal pressure, the barium acetate and strontium acetate that meet the stoichiometric ratio of the above chemical formula were dissolved in 120ml deionized water to obtain a clear and transparent solution with a concentration of Ba ions of 0.32M, containing 0.0016mol of Sr ions in the solution;
[0040] (2) Preparation of a solution containing Zr and Y ions
[0041] At room temperature and normal pressure, dissolve yttrium nitrate and zirconium nitrate in accordance with the stoichiometric ratio of the above chemical formula in 20ml of deionized water, and react to form a clear and transparent solution with a zirconium ion concentration of 0.08M and a yttrium ion concentration of 0.01M;
[0042] (3) Preparation of a solution containing Ti ions
[0043] At room temperature and norma...
Embodiment 2
[0051] Example 2: Ba 0.96 Sr 0.04 Ti 0.995 Zr 0.005 o 3 Preparation of micropowder
[0052] (1) Preparation of a solution containing Ba, Sr ions
[0053] At room temperature and normal pressure, the barium acetate and strontium acetate that meet the stoichiometric ratio of the above chemical formula were dissolved in 120ml deionized water to obtain a clear and transparent solution with a concentration of Ba ions of 0.32M, containing 0.0016mol of Sr ions in the solution;
[0054] (2) Preparation of a solution containing Zr ions
[0055] Under room temperature and normal pressure, zirconium nitrate conforming to the stoichiometric ratio of the above chemical formula is dissolved in 20ml of deionized water, and the reaction forms a clear and transparent solution with a zirconium ion concentration of 0.01M;
[0056] (3) Prepare a solution of Ti ions
[0057] At room temperature and normal pressure, dissolve tetrabutyl titanate conforming to the stoichiometric ratio of the a...
Embodiment 3
[0065] Example 3: Ba 0.96 Sr 0.04 Ti 0.895 Dy 0.005 Zr 0.10 o 2.9975 Preparation of micropowder
[0066] (1) Preparation of a solution containing Ba, Sr ions
[0067]At room temperature and normal pressure, the barium acetate and strontium acetate that meet the stoichiometric ratio of the above chemical formula were dissolved in 120ml deionized water to obtain a clear and transparent solution with a concentration of Ba ions of 0.32M, containing 0.0016mol of Sr ions in the solution;
[0068] (2) preparation contains the solution of Zr and Dy nitrate
[0069] At room temperature and normal pressure, dissolve zirconium nitrate and dysprosium nitrate in 20ml of deionized water in accordance with the above stoichiometric ratio, and react to form a clear and transparent solution with a zirconium ion concentration of 0.2M and a dysprosium ion concentration of 0.01M;
[0070] (3) Preparation of a solution containing Ti ions
[0071] At room temperature and normal pressure, dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com