Reductase, gene and recombinase of reductase and preparation method and application of recombinase
A reductase and gene technology, applied in the field of bioengineering, can solve the problems of low overall catalytic activity, narrow asymmetric reduction substrate spectrum, low enzyme yield, etc., to achieve strong stereoselectivity, wide substrate applicability, catalytic Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Cloning of embodiment 1 reductase gene
[0051] According to the gene sequence of the reductase YtbE, FabG and YueD of Bacillus subtilis (Bacillus subtilis) 168 that has been recorded in Genbank, the PCR primers were designed as follows:
[0052] Primer 1 (amplification of BCR I gene):
[0053] Upstream primer: CGC GGATCC ATGACAACACATTTACAAAGCAAAAG
[0054] Downstream primer: CCG GTCGAG TTAAAAATCAAAGTTGTCCGGATC
[0055] Primer 2 (amplification of BCR II gene):
[0056] Upstream primer: CGC GGATCC ATGCTTAATGATAAAACGGCT
[0057] Downstream primer: CCG GTCGAG TTACATCACCATTCCGCC
[0058] Primer 3 (amplification of BCR III gene):
[0059] Upstream primer: CGC GGATCC ATGGAACTTTATATCATCACCGGAG
[0060] Downstream primer: CCG GTCGAG CTACAAAAACTCTTTAATATCATAAATGCG
[0061] Wherein, the underlined part of the upstream primer is the BmHI restriction site, and the underlined part of the downstream primer is the XhoI restriction site.
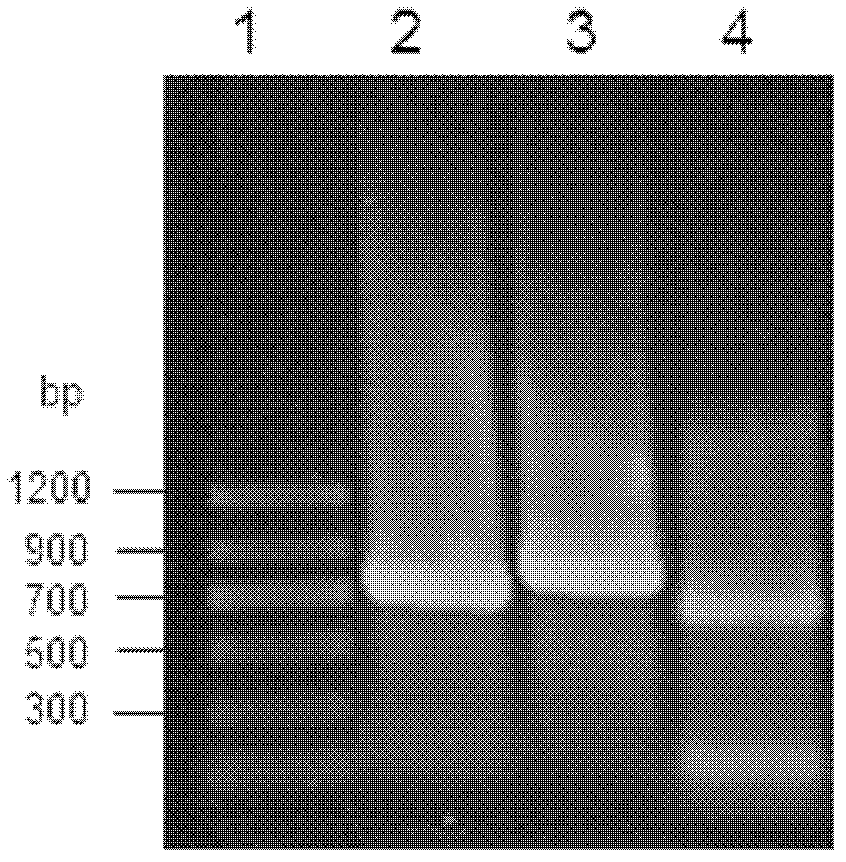
[0062] The genomic DNA of Ba...
Embodiment 2
[0066] Embodiment 2 Preparation of recombinant expression vector (plasmid) and recombinant expression transformant
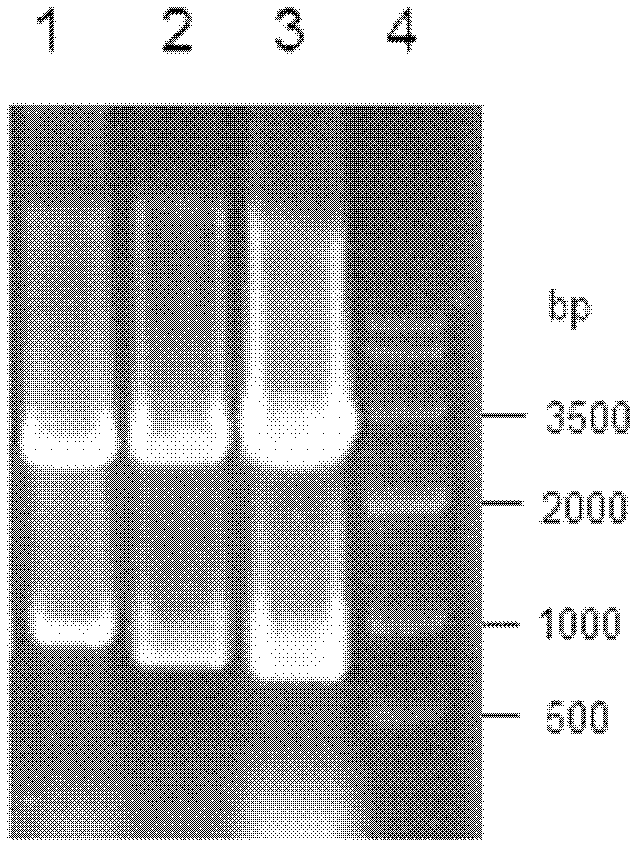
[0067] The reductase gene obtained in Example 1 was connected to the pMD-18T vector to construct cloning plasmids pBCRI-18T, pBCRII-18T and pBCRIII-18T, respectively. Afterwards, they were transformed into Escherichia coli (E.coli) DH5α competent cells, positive clones were screened by colony PCR, plasmids were extracted, and digested with restriction endonucleases BamHI and XhoI at 37°C for 12 hours, gelatinized in agarose Purify by gel electrophoresis, use the agarose gel DNA recovery kit to recover the target fragment, and prove that it contains the correct insert fragment ( figure 2 ). Under the action of T4 DNA ligase, the target fragment was ligated with the plasmid pET28a digested with BamHI and XhoI at 4°C overnight to obtain recombinant expression plasmids pET-BCRI, pET-BCRII and pET-BCRIII.
[0068] Transform the above-mentioned recombinant expressi...
Embodiment 3
[0069] Embodiment 3 expression of recombinant reductase
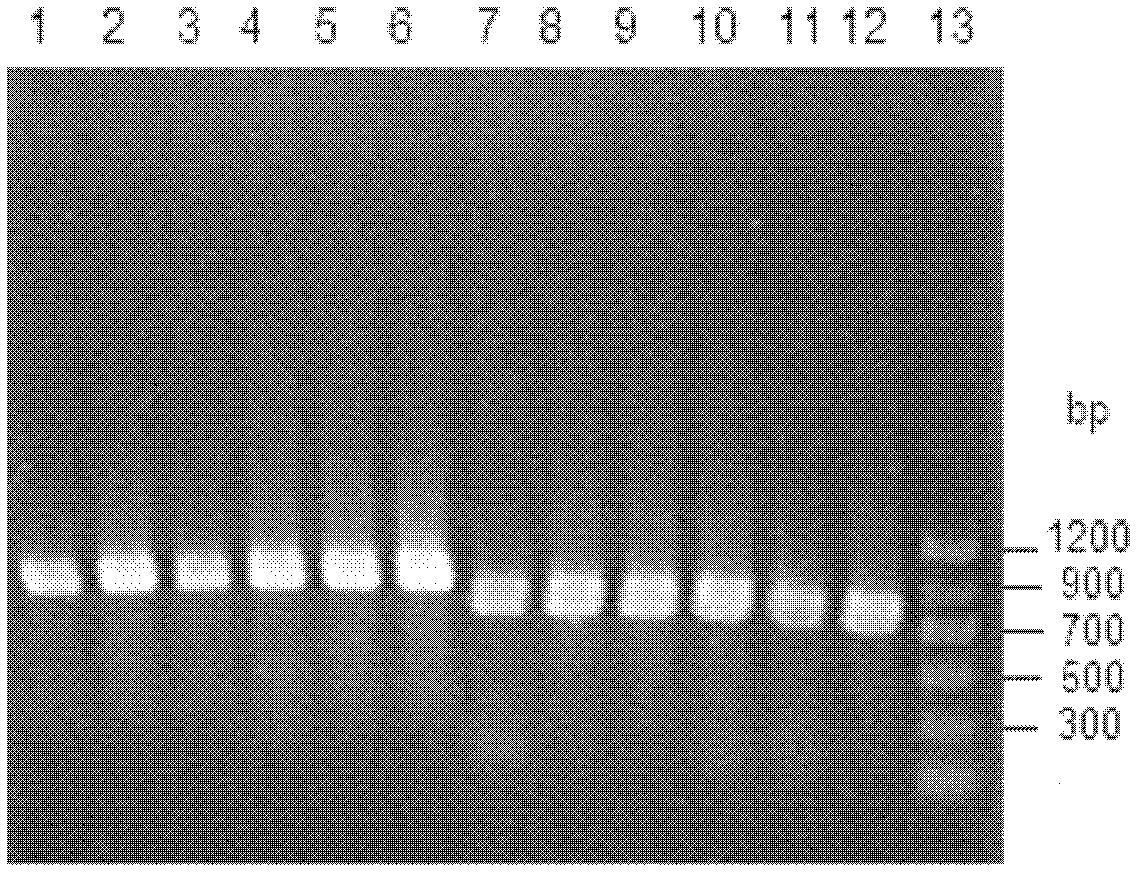
[0070] The 3 kinds of recombinant Escherichia coli obtained in Example 2 were inoculated into the LB medium containing ampicillin respectively, and cultured with shaking at 37°C overnight, and were inserted into 50ml LB medium ( Peptone 10g / L, yeast extract 5g / L, NaCl 10g / L, pH 7.0) in a 250ml Erlenmeyer flask, cultured on a shaker at 37°C and 180rpm, when the OD of the culture solution 600 When it reaches 0.6, add IPTG with a final concentration of 0.5mmol / L as an inducer, and after induction at 25°C for 12h, centrifuge the culture medium, collect the cells, wash twice with normal saline, and suspend the resulting resting cells in pH 7.0 buffer solution, sonicate in an ice bath, and centrifuge to collect the supernatant, which is the crude enzyme solution of the recombinant reductase. The crude enzyme solution was analyzed by polyacrylamide gel electrophoresis ( Figure 8 ), most of the three recombinant proteins exi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com