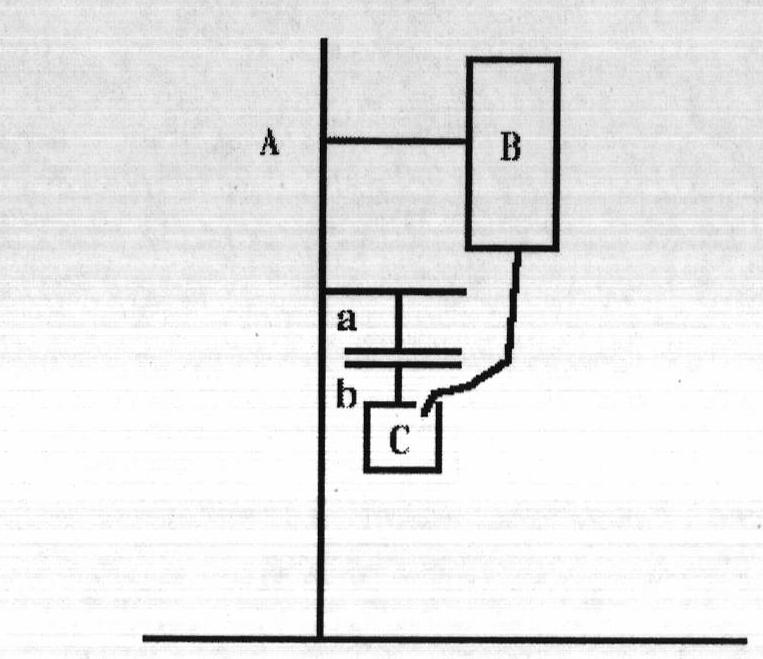
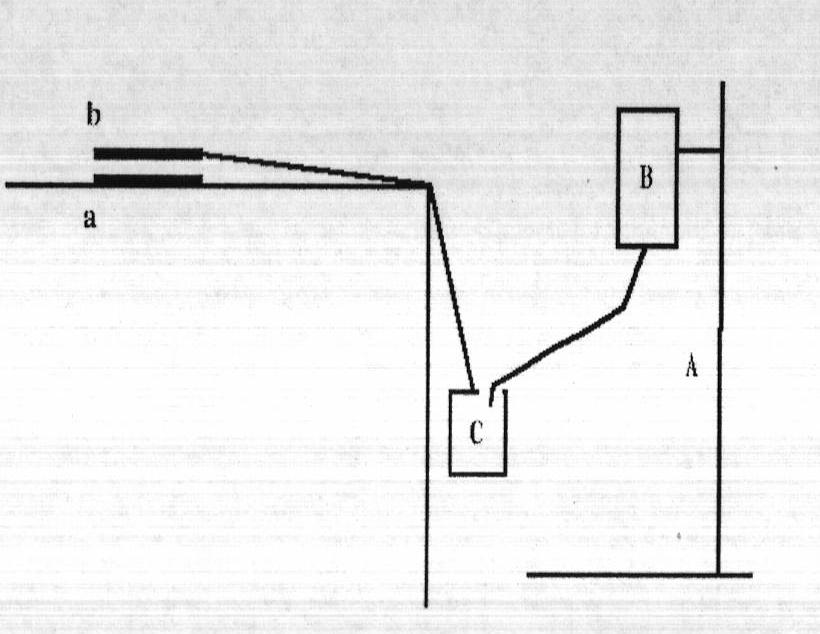
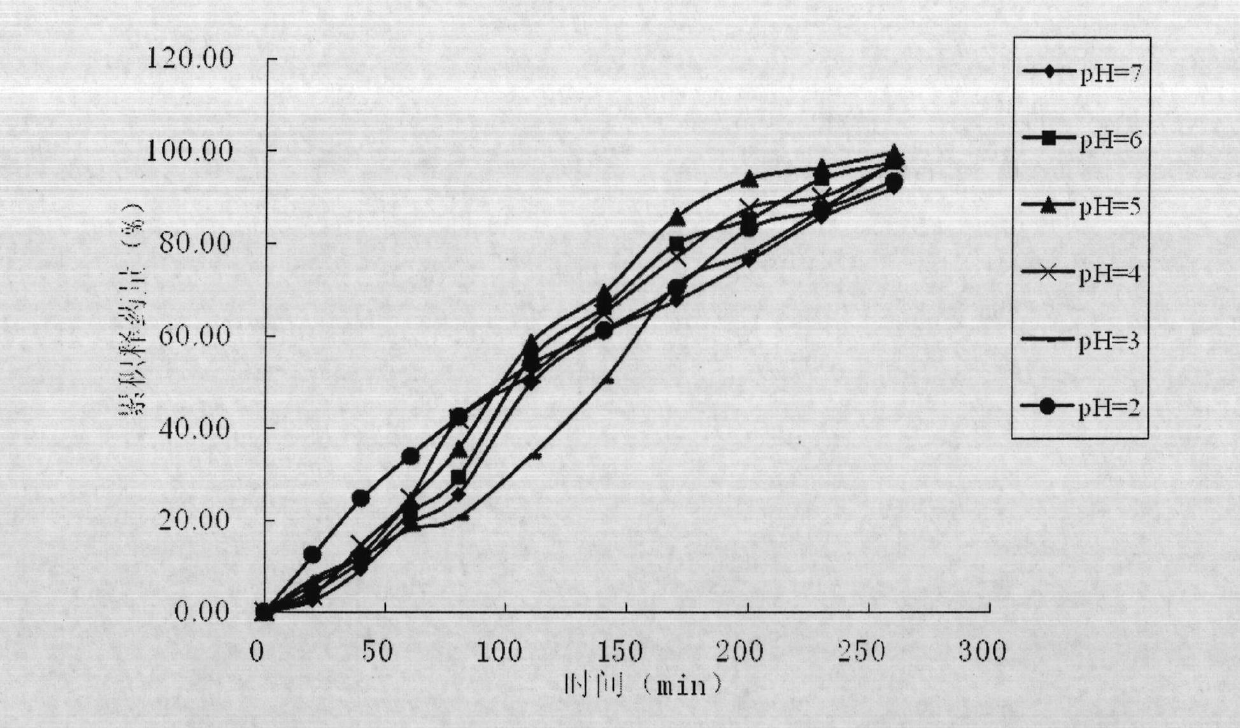
Bioadhesive vaginal plug for treating vaginitis and cervicitis as well as preparation method of bioadhesive vaginal plug
A bioadhesion and vaginal suppository technology, which is applied in the directions of medical preparations containing active ingredients, suppository delivery, drug combination, etc. Tissue crusting, repair, regeneration and healing, etc., to achieve the effect of not easily falling off, avoiding the physiological checkpoint effect of the gastrointestinal tract, and avoiding the first-pass effect of the liver
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Preparation of bioadhesive vaginal suppository matrix solution: Weigh 6.0g of gelatin and add it to 6mL of water for injection to make it fully swell, heat it in a water bath at 60°C, stir to dissolve, and form a gelatin colloidal solution, and then add 4.8g of glycerin, 5.4mL of polyvinyl alcohol-1788 solution with a concentration of 0.1g / mL and 3.0mL of N-succinyl chitosan solution with a concentration of 0.12g / mL were stirred and mixed evenly to obtain a bioadhesive pessary matrix Solution, keep warm at 40-50°C.
[0038] (2) Preparation of bioadhesive vaginal suppository: Take 1 mL of Sophora sophora total alkali solution with a concentration of 2 g / mL and 1 mL of external recombinant human basic fibroblast growth factor solution with a concentration of 100 IU / mL and add them to the matrix solution of bioadhesive vaginal suppository Stir, mix evenly, pour it into a duckbill pessary suppository mold coated with an appropriate amount of liquid paraffin while it is ...
Embodiment 2
[0040] (1) Preparation of bioadhesive vaginal suppository matrix solution: Take 6.6g of gelatin and add it to 5mL of water for injection to make it fully swell, heat it in a water bath at 60°C, stir to dissolve, and form a gelatin colloid solution, and then add 6.0 g of glycerin, 5 mL of polyvinyl alcohol-1788 solution with a concentration of 0.114 g / mL and 3 mL of N-succinyl chitosan solution with a concentration of 0.14 g / mL, stirred, and mixed uniformly to obtain a bioadhesive vaginal suppository matrix solution, 40 ~50°C heat preservation.
[0041] (2) Preparation of bioadhesive vaginal suppository: Take 1 mL of Sophora sophora total alkali solution with a concentration of 2 g / mL and 0.5 mL of external recombinant human basic fibroblast growth factor solution with a concentration of 1000 IU / mL and add them to the matrix of the bioadhesive vaginal suppository solution, stirred, mixed evenly, poured into a duckbill-shaped pessary suppository mold coated with an appropriate a...
Embodiment 3
[0043] (1) Preparation of bioadhesive vaginal suppository matrix solution: Take 5.4g of gelatin and add it to 6mL of water for injection to make it fully swell, heat it in a water bath at 60°C, stir to dissolve, and form a gelatin colloid solution, then add 5.4 g of glycerin, 5 mL of polyvinyl alcohol-1788 solution with a concentration of 0.102 g / mL and 3 mL of N-succinyl chitosan solution with a concentration of 0.10 g / mL, stirred, and mixed uniformly to obtain a bioadhesive vaginal suppository matrix solution, 40 ~50°C heat preservation.
[0044] (2) Preparation of bioadhesive vaginal suppository: Take 1 mL of total alkali solution of Sophora sophorae with a concentration of 1.4 g / mL and 2 mL of external recombinant human basic fibroblast growth factor solution with a concentration of 750 IU / mL and add them to the above-mentioned bioadhesive vaginal suppository matrix solution, stirred, mixed evenly, poured into a duckbill-shaped pessary suppository mold coated with an appro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com