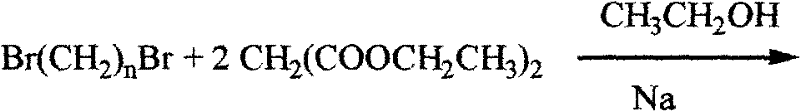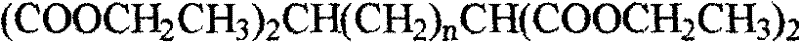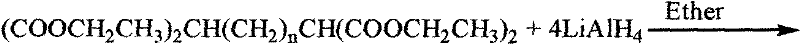Tetra-azido alkane and preparation method thereof
A technology of tetraazide-based alkanes and nitrogen-based alkanes, which is applied in the field of tetraazide-based alkanes and their preparation, can solve the problems that the preparation technology cannot be widely popularized and applied, and the burning speed is slow, and the synthesis process is simple and stable, and the burning speed is fast. , good speed-up performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] The principle of the synthesis method of the tetraazide alkane of the present invention is that the optional alkali metal azide compounds include lithium azide, potassium azide and sodium azide, among which sodium azide is preferred. The separation of the product is carried out by column chromatography on a silica gel column to obtain the azide product.
[0011] Concrete synthetic route is as follows:
[0012]
[0013]
[0014]
[0015]
[0016]
[0017]
[0018]
[0019]
[0020] where n=2, 3, 4, 5 or 6.
[0021] Synthetic method of the present invention has the following steps:
[0022] 1 Preparation and characterization of tetraethyl 1,1,5,5-pentane tetracarboxylate
[0023] Under nitrogen protection, add 3.45g of sodium block into 75mL of absolute ethanol and dissolve at low temperature (-10°C-10°C). Reflux until the reaction is complete, and finally add 5.1 mL of 1,3-dibromopropane dropwise to the mixed solution. After the dropwise additi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com