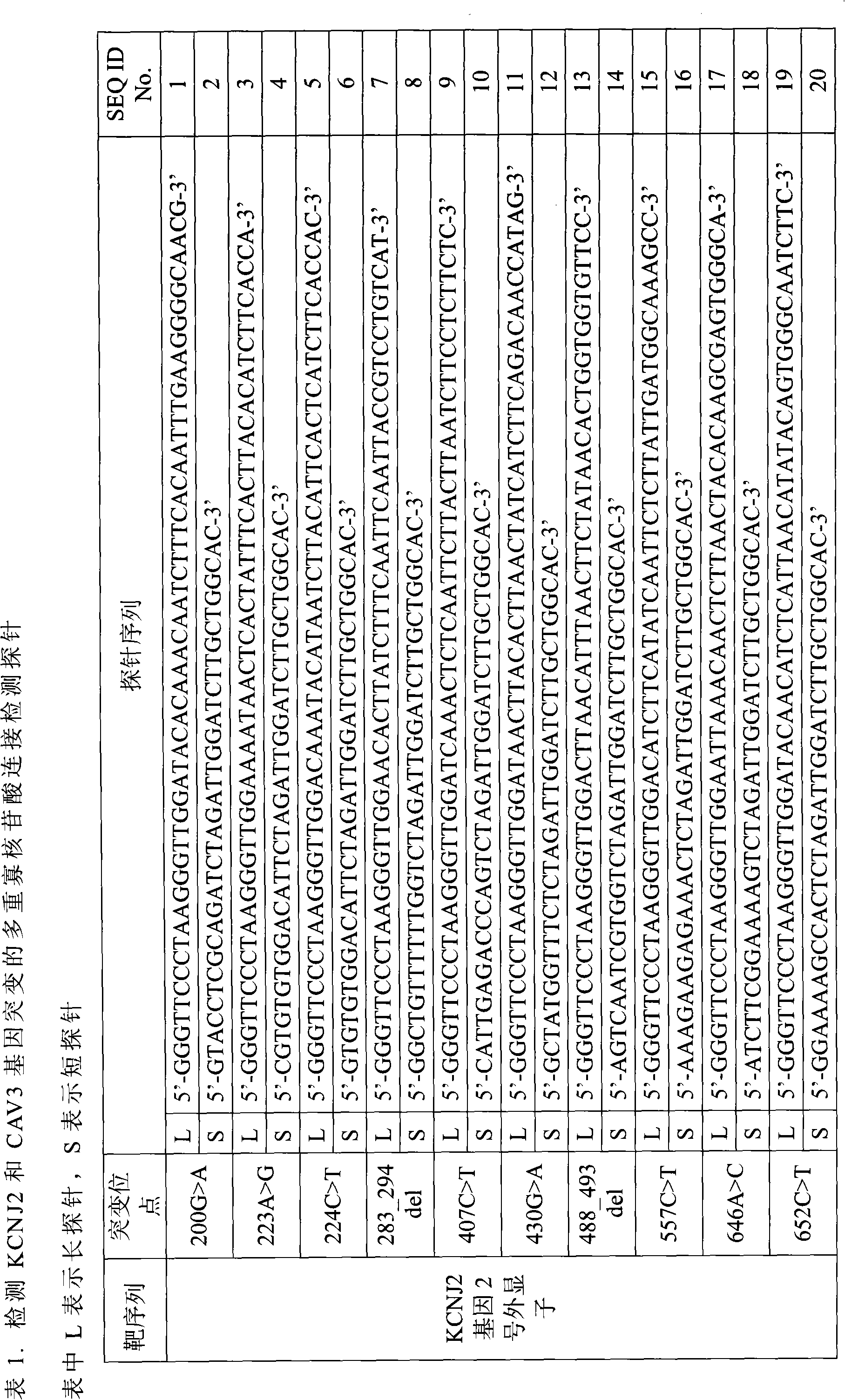
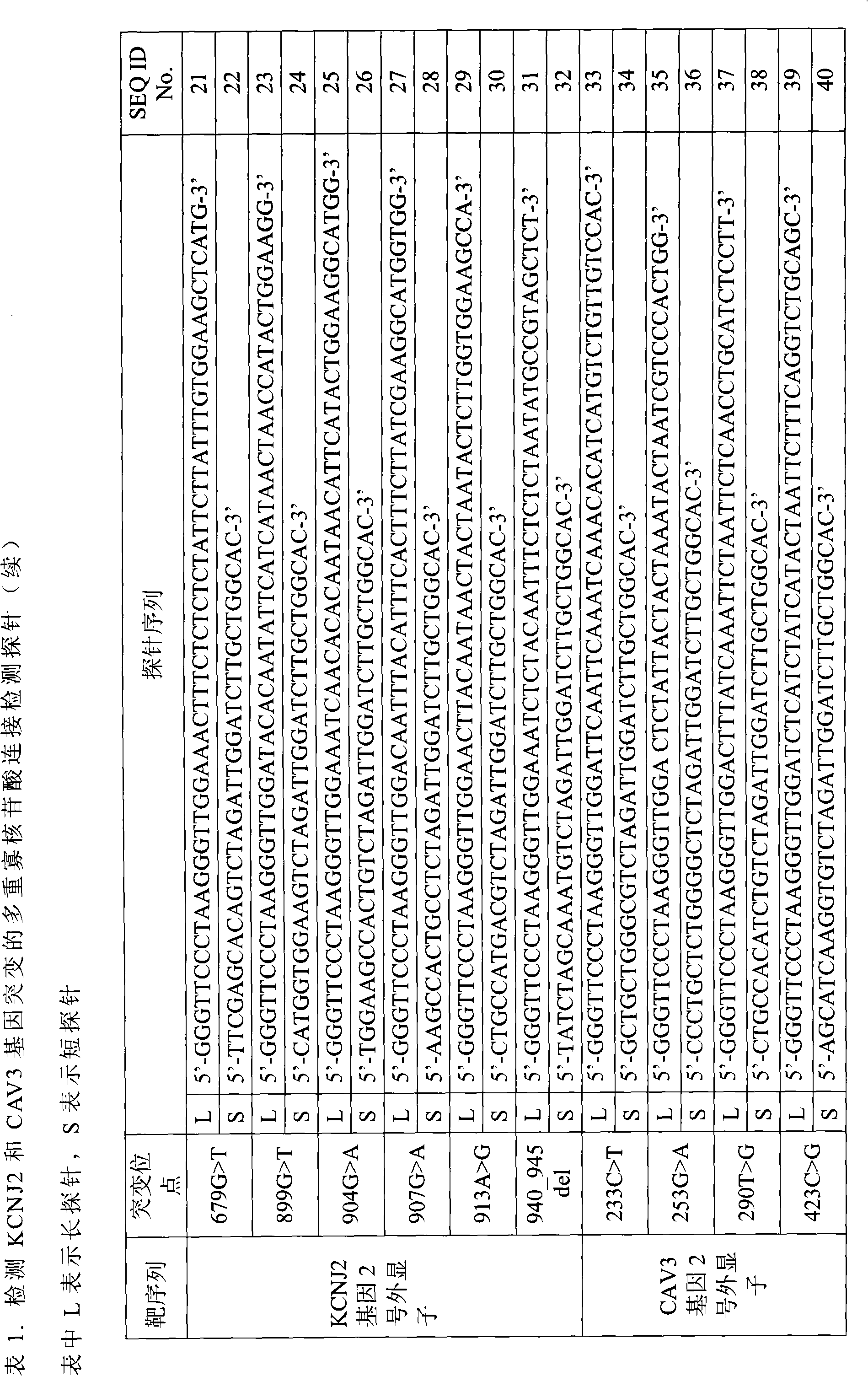
Method for detecting mutation of related genes of genetic long QT syndrome
A gene and ligation detection technology, applied in the field of molecular biology, can solve the problems of inability to achieve high throughput, affect the detection results, complex steps, etc., to improve detection accuracy and stability, avoid laboratory contamination, and simple steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, extract the DNA of the sample to be tested according to conventional methods
[0043] Take the sample to be tested (including whole blood, plasma, and serum), and extract the DNA in the sample according to the instructions of the Kangwei Century Blood Genome Small Extraction Kit. The detailed steps are as follows:
[0044] 1) Add 20μl Protease to the bottom of a 1.5ml centrifuge tube.
[0045] 2) Add 200μl sample
[0046] 3) Add 200 μl Buffer GL to the sample, and vortex for 15 seconds. Add 20ul DNase-free RNaseA.
[0047] 4) Incubate at 56°C for 10 minutes.
[0048] 5) Add 200 μl of absolute ethanol, vortex for 15 seconds, centrifuge briefly after mixing, so that the liquid on the tube wall and the cap is concentrated at the bottom of the tube.
[0049] 6) Put the ready-to-use Spin Column DM into the Collection Tube, carefully transfer the solution obtained in step 5) into the Spin Column DM, and avoid adding to the edge of the Spin Column DM, cover t...
Embodiment 2
[0056] Example 2, DNA sample denaturation and probe hybridization, connecting the probes, and performing PCR amplification on the connected probe sequences
[0057] 1) Configure the reaction system. Since multiple ligation and amplification reactions are carried out in a single tube, add the DNA sample and probe to be tested into the reaction tube, and then add the required reagents for the ligation reaction and PCR amplification reaction into the tube. Each 10μl reaction system includes: 0.8μl DNA ligase (5U / μl), 1μl long probe mixed solution (50nM), 1μl short probe mixed solution (50nM), 1μl Buffer II (10×), 0.8μl 4 kinds of dNTPs mixed solution (2.5mM), 1.6μl magnesium chloride (25mM), 0.1μl DNA polymerase, 0.1μl nicotinamide adenine dinucleotide (50mM), 0.2μl biological Primer-labeled forward universal primer (10 mM), 0.2 μl reverse universal primer (10 mM), 1 μl DNA template extracted according to Example 1, and finally add sterile double distilled water until the entire...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com