Application of combination of tetracycline medicine and fluconazole in preparation of antifungal product, and product thereof
A fluconazole and antifungal technology, applied in the field of medicine, can solve problems such as difficulty in clinical treatment of fungal infections, and achieve strong effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
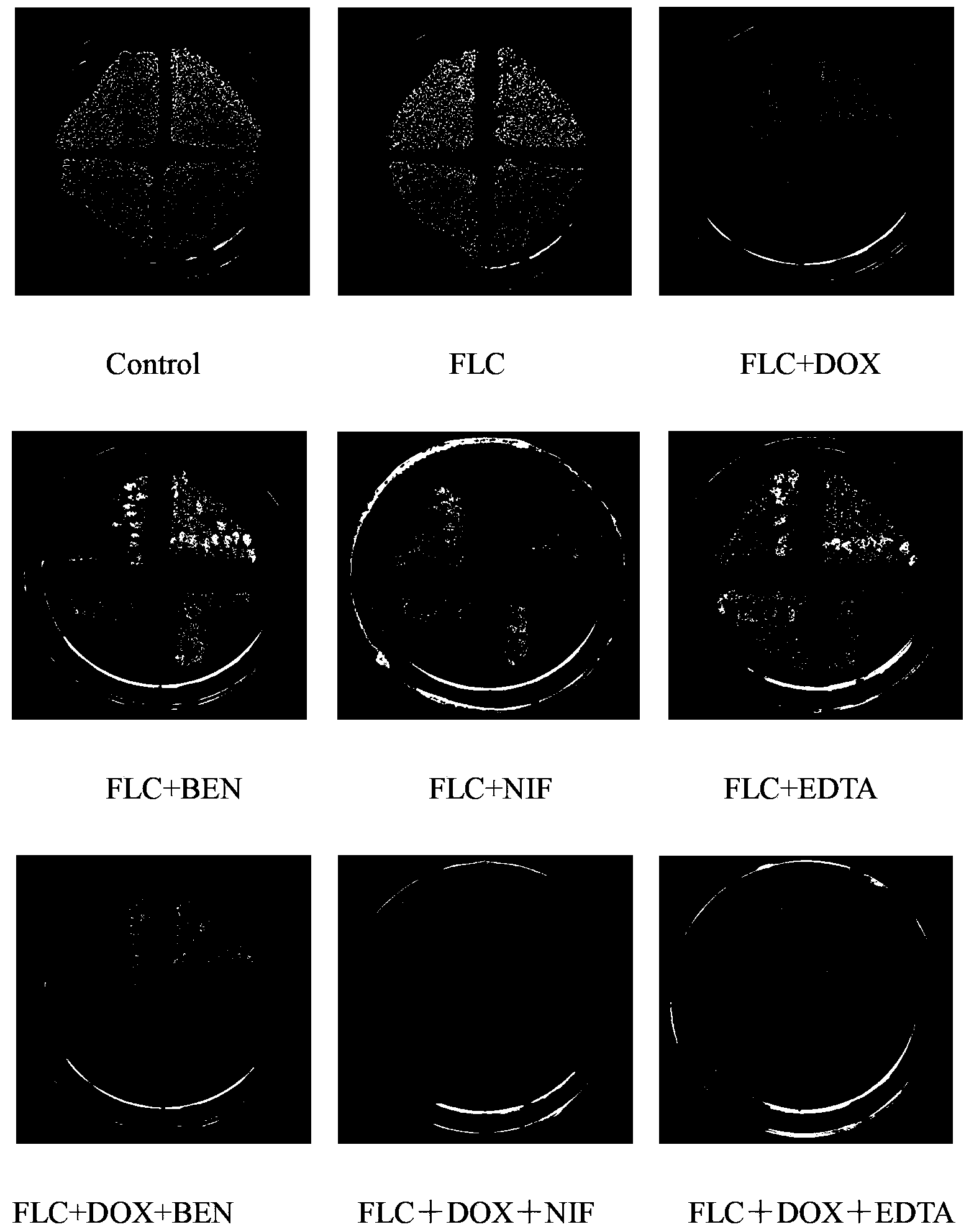
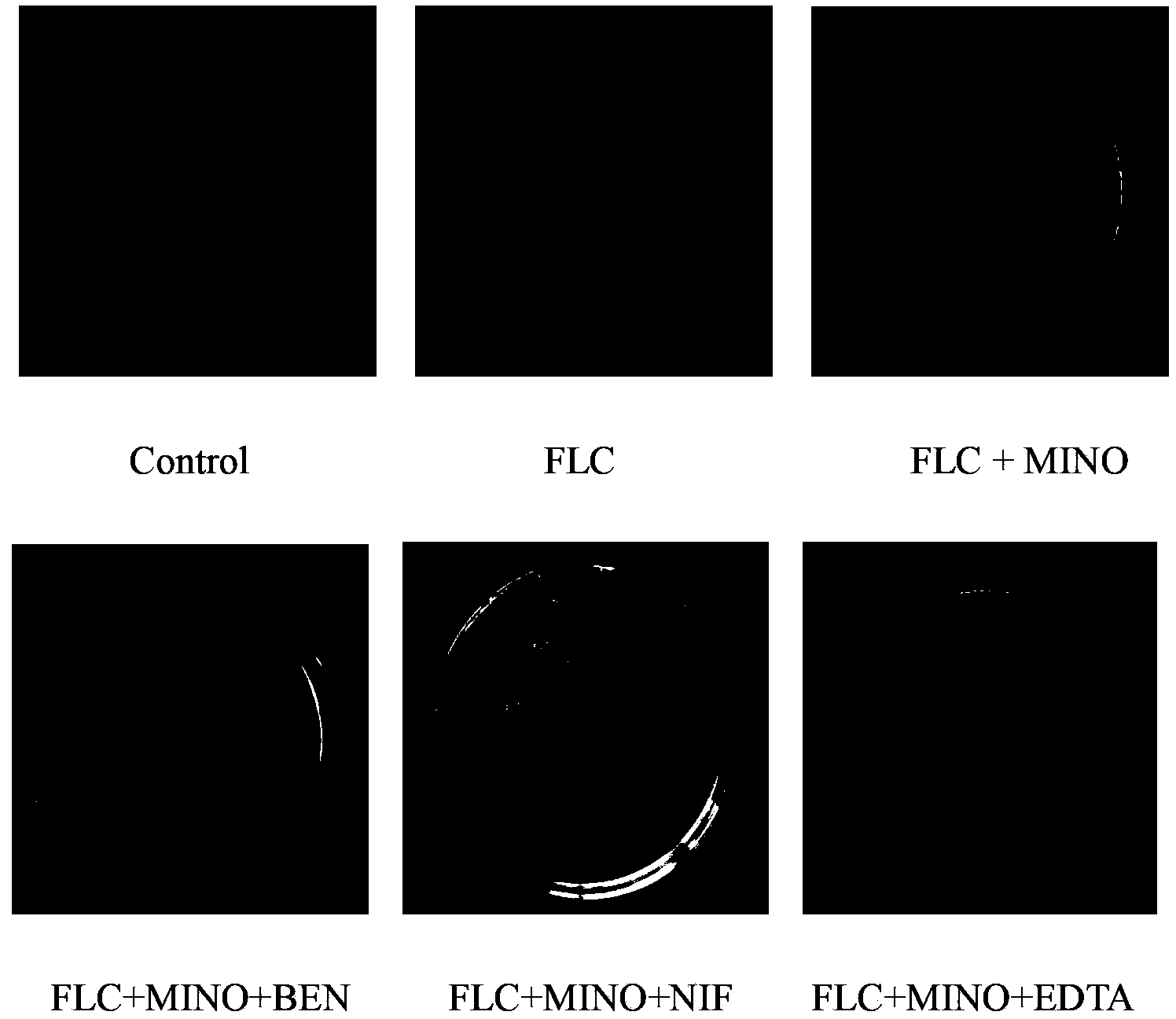
[0038] Embodiment 1 FLC and DOX / MINO combined antifungal action assay
[0039] 1. Materials
[0040] 1.1 Drugs and reagents
[0041] Fluconazole (FLC), Shandong Chengchuang Pharmaceutical Technology Development Co., Ltd.;
[0042] Minocycline (minocycline, MINO), minocycline hydrochloride, Shandong Panxin Biotechnology Co., Ltd.;
[0043] Doxycycline (DOX), Shandong Drug Testing Institute;
[0044] CHROMagar Candida Chromogenic Medium, Zhengzhou Bosai Biological Engineering Co., Ltd.;
[0045] TTC-Sapauer agar, Hangzhou Tianhe Microbiological Reagent Co., Ltd.;
[0046] Sodium hydroxide, state-owned Shandong Shanxian Organic Chemical Factory, batch number 940420;
[0047] Potassium dihydrogen phosphate, Shanghai Xinbao Fine Chemical Factory, batch number 200602132.
[0048] Dimethyl sulfoxide (DMSO), China Yaoshun Import and Export Co., Ltd., batch number 031012;
[0049] RPMI 1640 API powder, American GIBCO company;
[0050] 3-(N-morpholino)propanesulfonic acid (MOPS)...
Embodiment 2
[0085] The influence of embodiment 2 calcium regulators on FLC and DOX / MINO antifungal action
[0086] 1. Materials
[0087] 1.1. Drugs and reagents
[0088] Benidipine (benidipine hydrochloride, BEN), Huaxia Pharmaceutical Co., Ltd.;
[0089] EDTA, No.1 Reagent Factory, Shanghai, China;
[0090] Nifedipine (Nifedipine, NIF), Shandong Provincial Drug Testing Institute;
[0091] Doxycycline (DOX), National Institute of Drug Control;
[0092] Drug stock solution: all the following drug solutions are stored in a -20°C refrigerator for later use.
[0093] Benidipine was made into 2560μg / ml stock solution in DMSO
[0094] Minocycline was made into 6400μg / ml stock solution in DMSO
[0095] Fluconazole was made into 2560μg / ml stock solution with sterile distilled water
[0096] Doxycycline was made into 6400 μg / ml stock solution with sterile distilled water
[0097] Nifedipine was made into 6400 μg / ml stock solution with sterile distilled water
[0098] EDTA was made into 0....
Embodiment 3
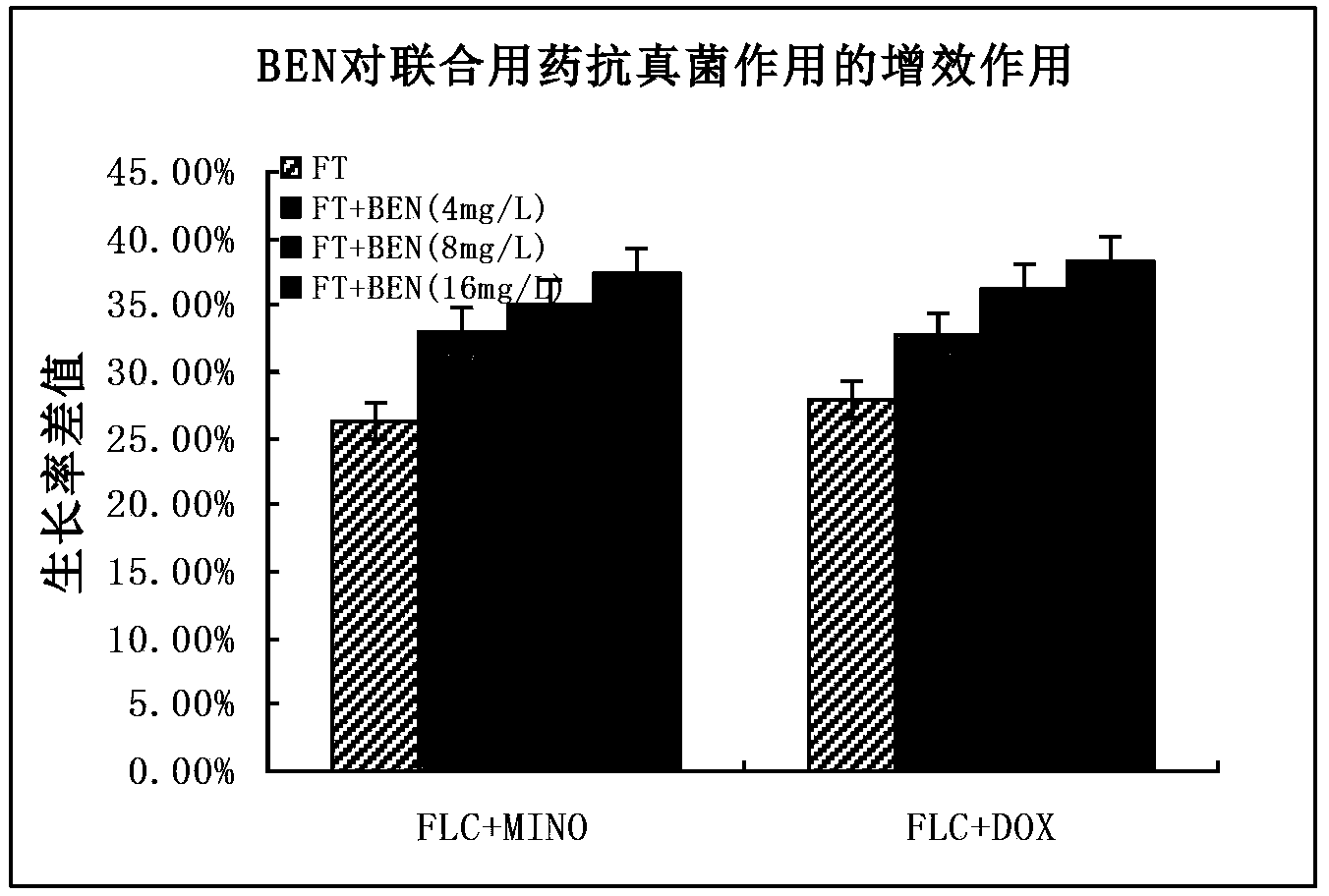
[0120] Quantitative Determination of Example 3 Calcium Regulators to the Synergistic Effect of Combined Drugs
[0121] 1. Materials
[0122] 1.1 Drugs and reagents
[0123] Benidipine (benidipine hydrochloride, BEN), Huaxia Pharmaceutical Co., Ltd.;
[0124] EDTA, No.1 Reagent Factory, Shanghai, China;
[0125] Nifedipine (Nifedipine, NIF), a gift from the Pharmacology Laboratory of Huazhong University of Science and Technology;
[0126] Doxycycline (DOX), National Institute of Drug Control;
[0127] Drug stock solution: all the following drug solutions are stored in a -20°C refrigerator for later use.
[0128] Benidipine was made into 2560μg / ml stock solution in DMSO
[0129] Minocycline was made into 6400μg / ml stock solution in DMSO
[0130] Fluconazole was made into 2560μg / ml stock solution with sterile distilled water
[0131] Doxycycline was made into 6400 μg / ml stock solution with sterile distilled water
[0132] Nifedipine was made into 6400 μg / ml stock solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com