Compounds including indene and difluoroethylene bridge bond, preparation method thereof and application thereof
A compound and reaction technology, applied in the compound containing indene ring and difluoroethylene bridge and its preparation and application field, can solve the problems of unsatisfactory liquid crystal materials, performance not suitable for market development, etc., and achieve large optical anisotropy , The synthesis route is simple and easy, and the effect of wide temperature range of nematic phase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
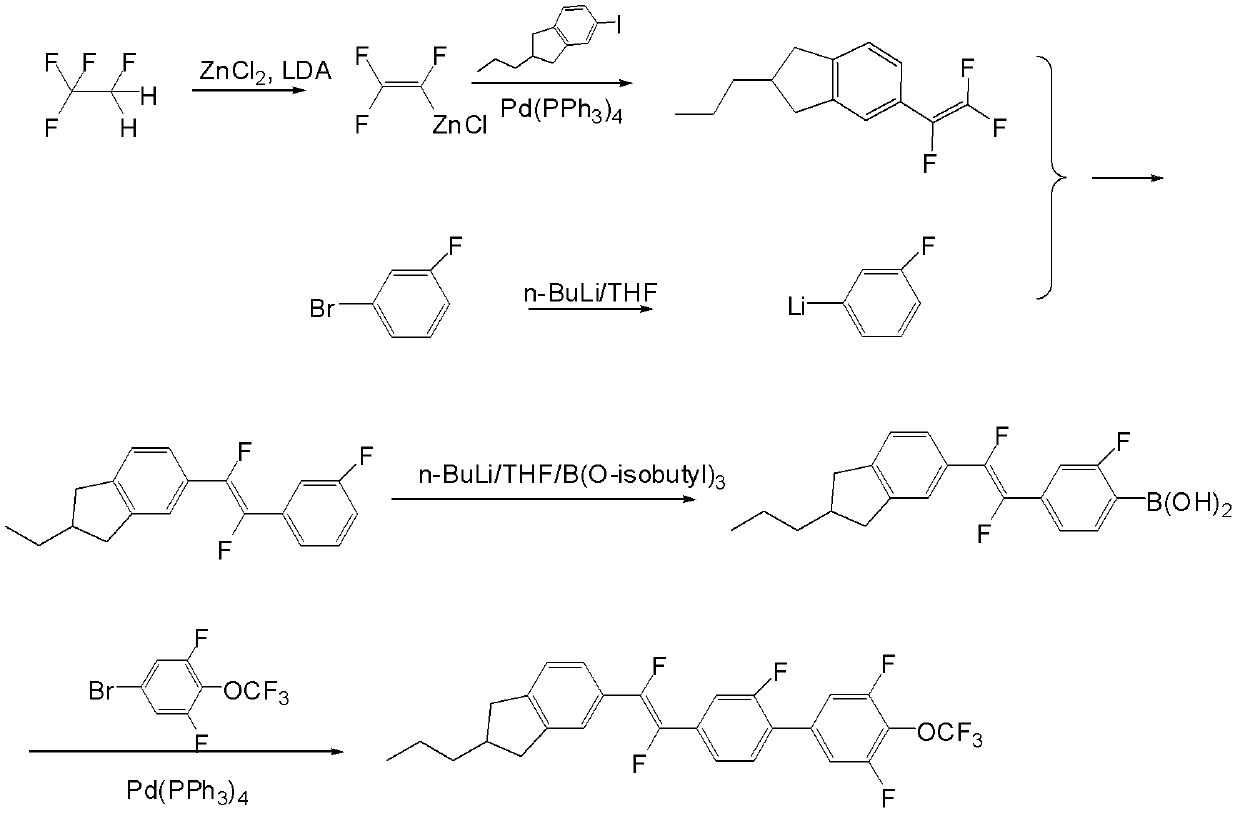
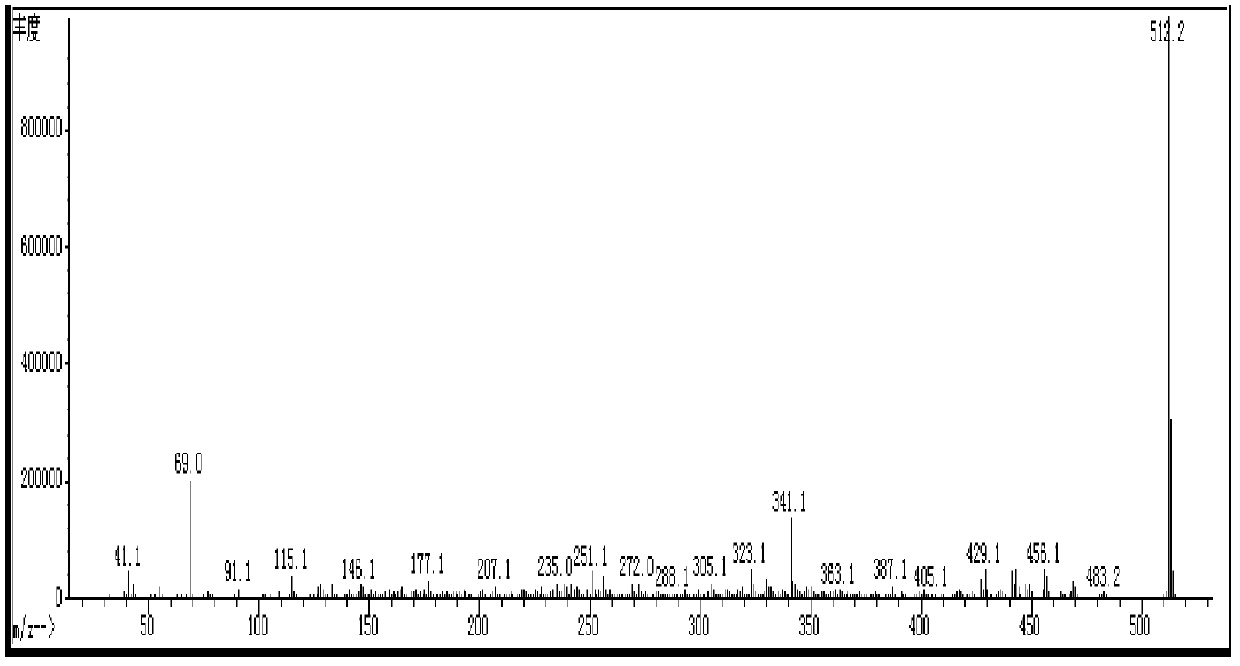
[0056] The synthetic route of the prepared compound I-3-1 is in figure 1 Said in, its specific process steps are as follows:
[0057]
[0058] 1) Synthesis of lithium diisopropylamide (LDA)
[0059] Add 115mL of diisopropylamine and 100mL of tetrahydrofuran (THF) into a 1000mL three-necked flask, under nitrogen protection, and add 320mL of n-butyllithium (n-BuLi, 2.4mol / L) dropwise under temperature control at 0°C to -20°C. Stir at ~-20°C for 1 hour to prepare LDA.
[0060] 2) Synthesis of trifluorovinyl zinc chloride
[0061]
[0062] Add 53g of anhydrous zinc chloride and 100mL THF to a 1000mL three-necked flask, protect it with nitrogen, cool down to -70°C, inject 54g of 1,1,1,2-tetrafluoroethane gas, and control the temperature from -50°C to -70°C Slowly inject LDA under the liquid surface through the needle tube, and stir for 2 hours after the addition, to obtain trifluorovinyl zinc chloride.
[0063] 3) compound of synthetic formula 6
[0064] (Formula 6)
...
Embodiment 2
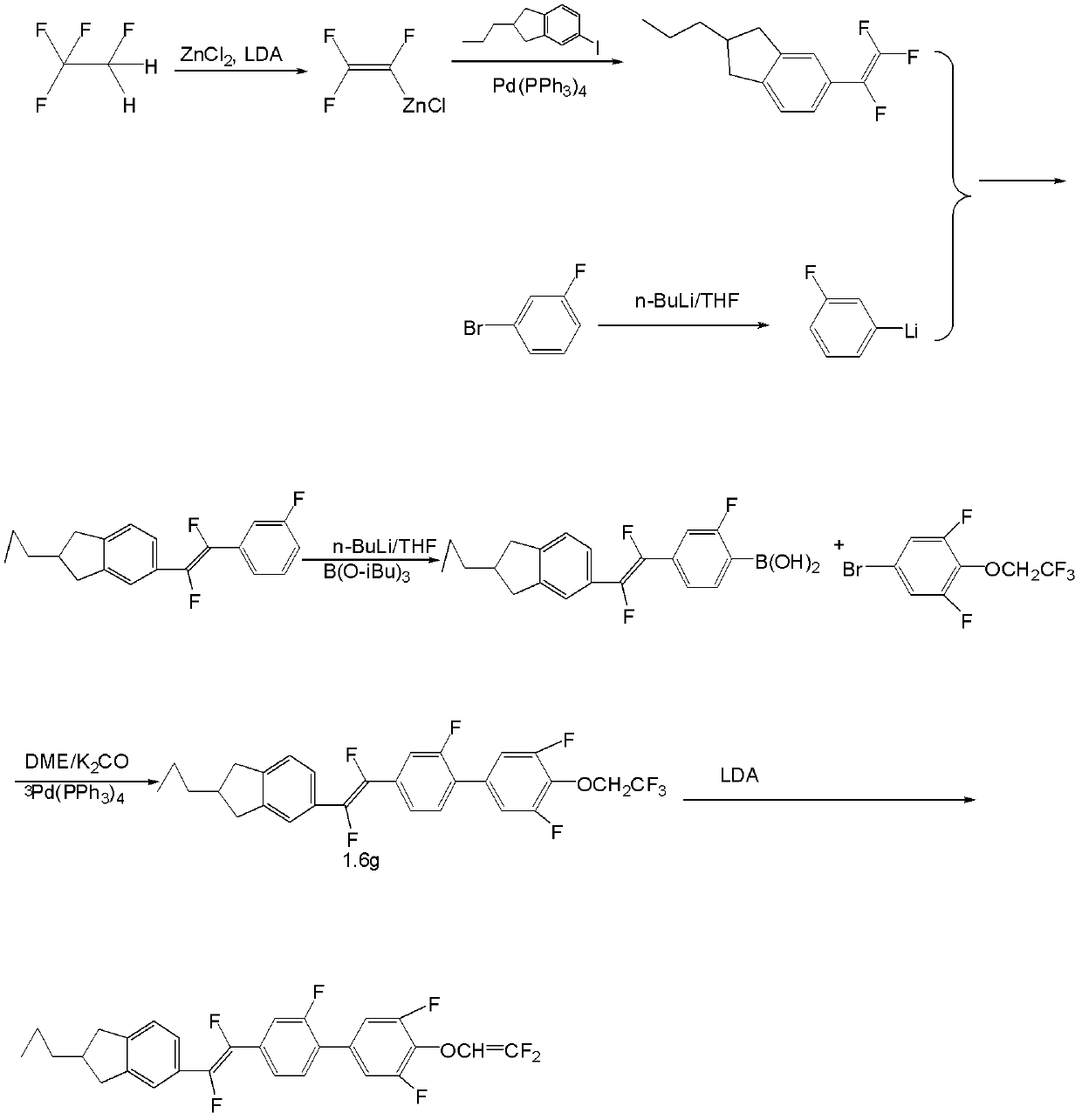
[0083] The synthetic route of the prepared compound I-3-2 is in image 3 Said in, its specific process steps are as follows:
[0084]
[0085] 1) Prepare the compound of formula 9 with steps 1) to 5) in Example 1.
[0086] 2) compound of synthetic formula 10
[0087] Formula 10
[0088] Take a dry 50ml single-necked bottle, add a magnetic stirrer, connect a condenser, add 1.75g of 3,5-difluoro-4-(2,2,2-trifluoroethaneoxy)bromobenzene, 5ml of 2mol / L potassium carbonate Aqueous solution, 0.5g tetrabutylammonium bromide, 15ml ethylene glycol dimethyl ether (DME), stirring, 1.6g compound of formula 9, 0.01g tetrakis (triphenylphosphine) palladium, nitrogen protection, reflux at 80°C for 3h .
[0089] The reaction solution was poured into 50 mL of water, extracted with ethyl acetate, the organic layers were combined, washed with water, and the solvent was evaporated under reduced pressure. Silica gel column chromatography with petroleum ether as eluent. Beat with absol...
Embodiment 3
[0097]The synthetic route of the prepared compound I-5-1 is in Figure 5 Said in, its specific process steps are as follows:
[0098]
[0099] 1) Prepare the compound of formula 6 with steps 1) to 3) in Example 1.
[0100] 2) compound of synthetic formula 11
[0101] Formula 11
[0102] Add 2.9g of 3.5-difluorobromobenzene and 50ml of tetrahydrofuran to a 100ml three-necked flask, protect with nitrogen, cool down to -78°C, slowly add 7.5ml of 2.4mol / L n-butyllithium dropwise, and react for 2 hours at a temperature controlled below -70°C , to prepare m-fluorophenyllithium reagent.
[0103] Dissolve the obtained compound of formula 6 (14 g) in 20 mL of tetrahydrofuran, control the temperature below -70°C, drop it into the m-fluorophenyllithium reagent prepared above, and react overnight at room temperature.
[0104] After the reaction was complete, the reaction was quenched with dilute glacial hydrochloric acid water, extracted with ethyl acetate, washed with water, dri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com