Solid-phase synthesis method for magnesium borohydride ammoniates
The technology of magnesium borohydride ammine compound and magnesium borohydride solid phase is applied in the field of preparation of hydrogen storage materials, and can solve the problems of carcinogenicity of benzene, low purity, difficult operation, etc. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
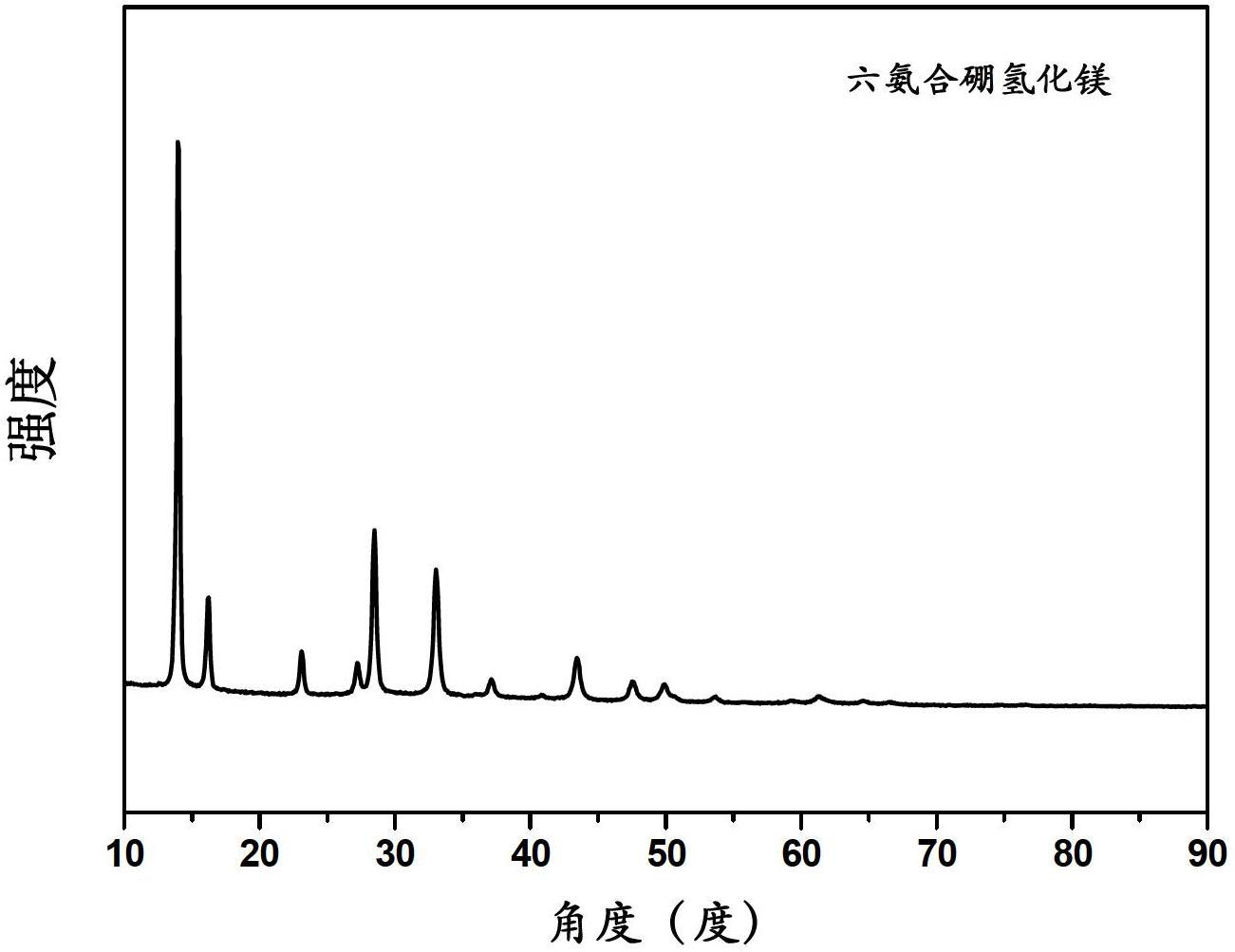
[0038] In the glove box, the Mg(BH 4 ) 2 Put it into a stainless steel ball mill tank that can be sealed and have a switch valve. After the ball mill tank is evacuated, fill it with ammonia with a pressure of 10 atmospheres (if there is no special description in the present invention, the atmospheric pressure refers to standard atmospheric pressure). , ball milling on a planetary ball mill, the ball-to-material ratio is 120:1, the rotating speed is 300rpm, and the ball milling time is 36h. After the ball milling is finished, the product is subjected to XRD testing (the XRD of the sample is measured on a Philips X'Pert Pro X-ray diffractometer ).
[0039] figure 1 It is the XRD pattern of the product, and the product has and only has the characteristic diffraction peak of hexaammine magnesium borohydride.
Embodiment 2
[0041] Magnesium borohydride ammonium was prepared according to the method described in Example 1, and the differences in preparation conditions are shown in Table 1.
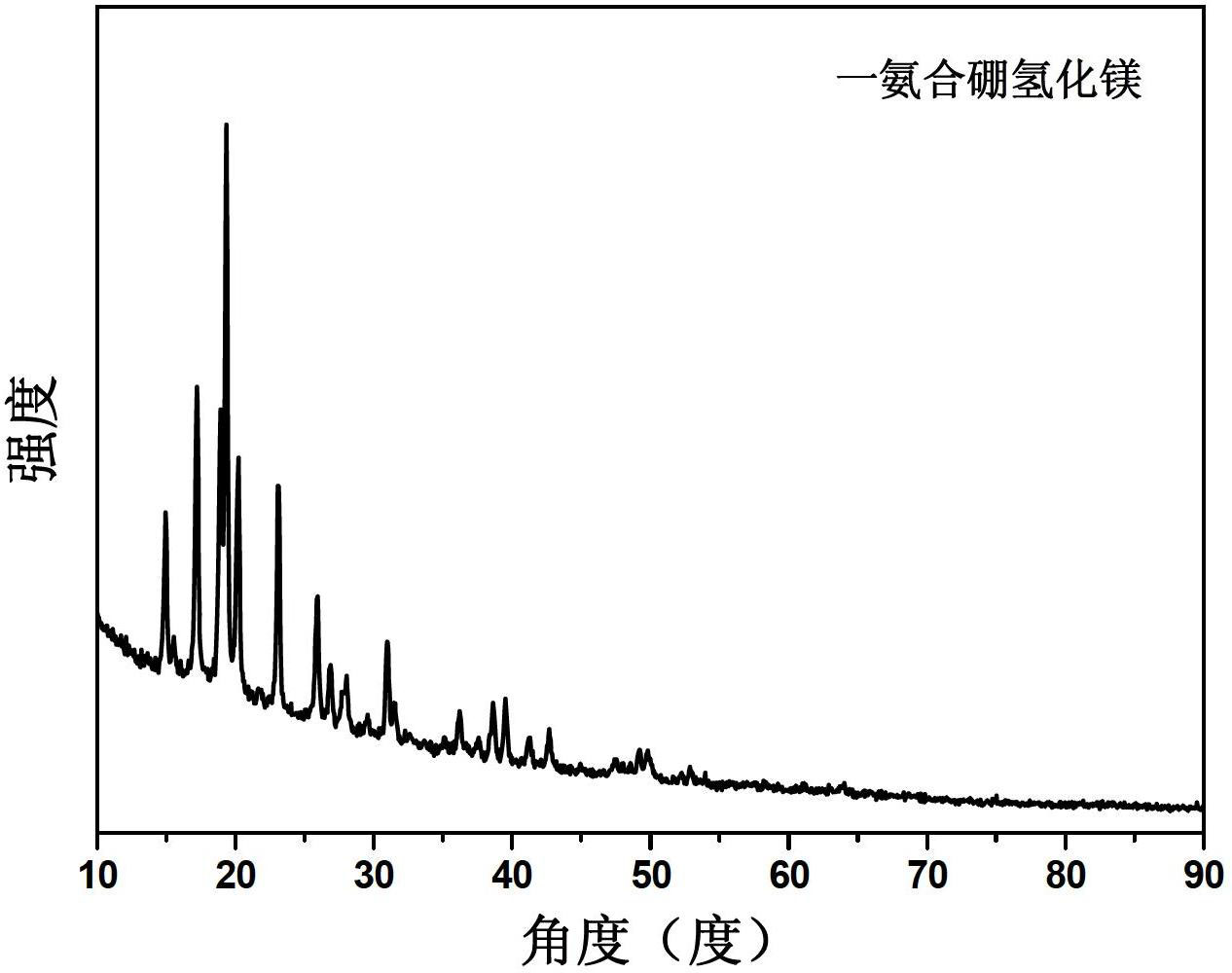
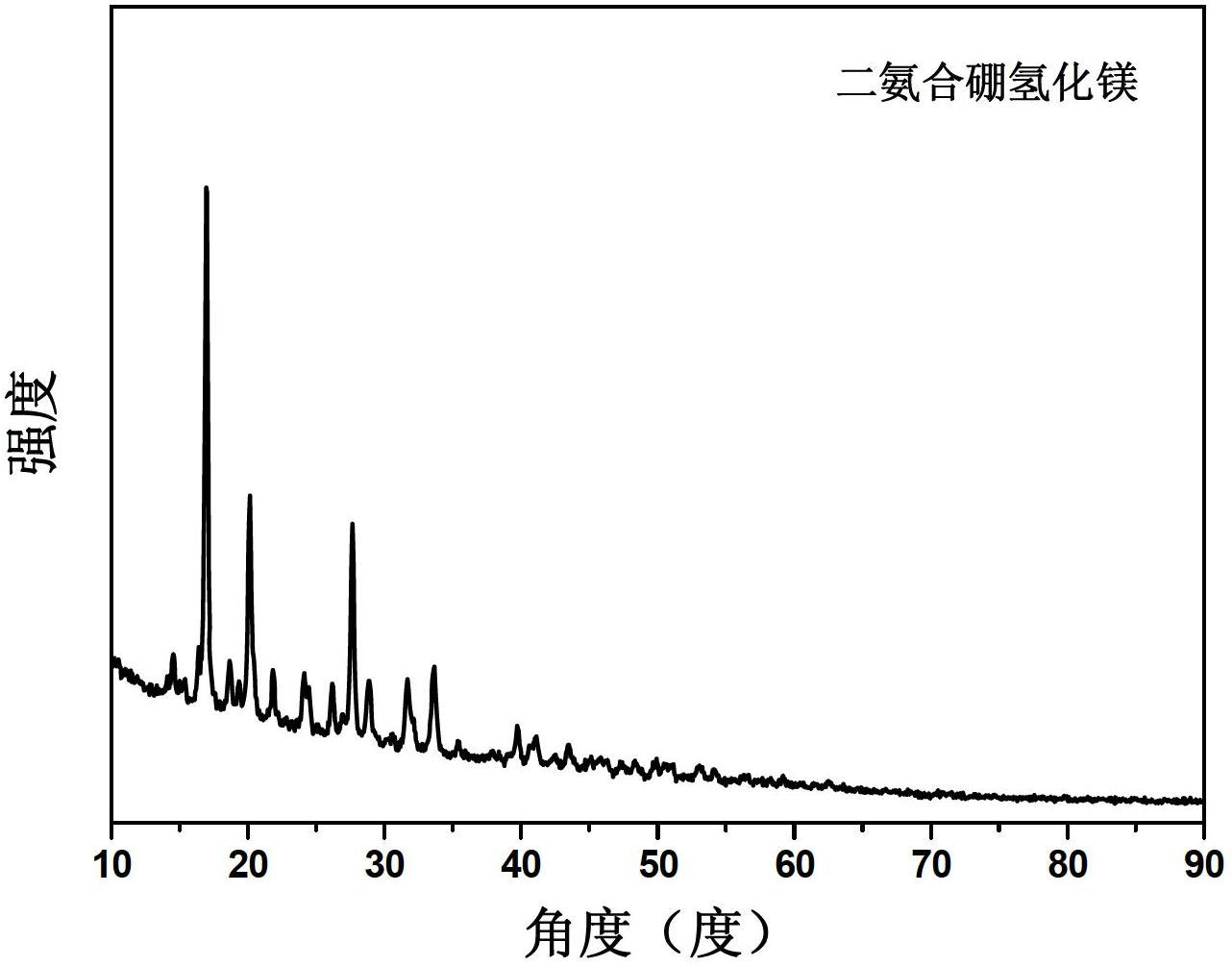
[0042] After the preparation, the product was weighed, and the ammonium complex number of magnesium borohydride in the product was calculated. Table 1 lists the ratio of product weight increase and the type of product. It was found that by changing the preparation conditions, monoammine magnesium borohydride, diammine magnesium borohydride, triammine magnesium borohydride, tetraammine magnesium borohydride and pentaammine magnesium borohydride can be obtained respectively.
[0043] Table 1 The experimental conditions and products of ball milling magnesium borohydride under ammonia atmosphere
[0044]
Embodiment 3
[0046]In the glove box, the Mg(BH 4 ) 2 and Mg(BH 4 ) 2 6NH 3 Mix according to the molar ratio of 5:1, put it into a stainless steel ball mill tank that can be sealed and have a switch valve, and ball mill it on a planetary ball mill under an argon atmosphere of 1 atmosphere pressure, the ball-to-material ratio is 60:1, and the speed is 550rpm. The time is 18h. After the ball milling, the product was tested by XRD.
[0047] figure 2 It is the XRD pattern of the product, and the product has and only one characteristic diffraction peak of ammine magnesium borohydride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com