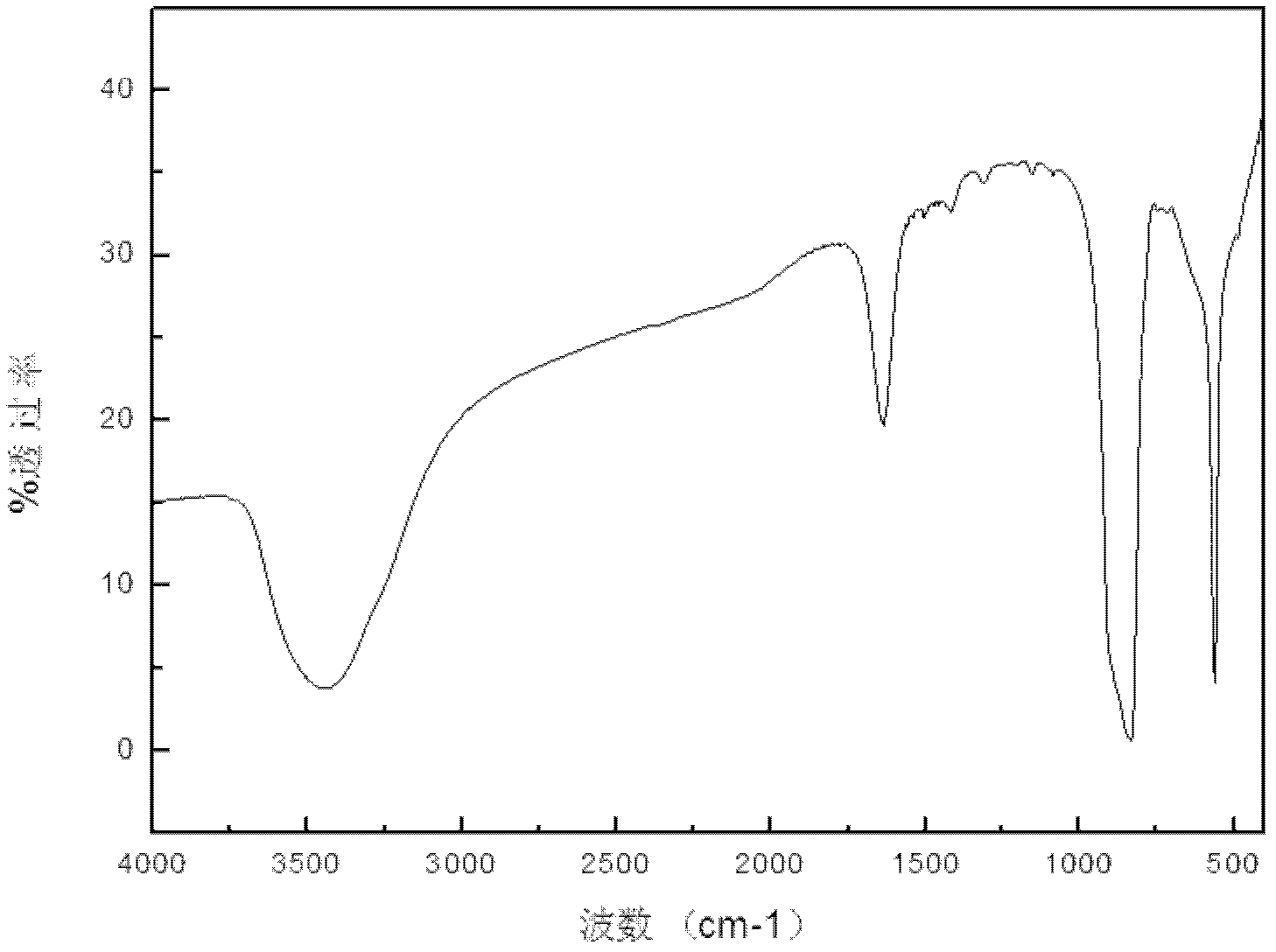
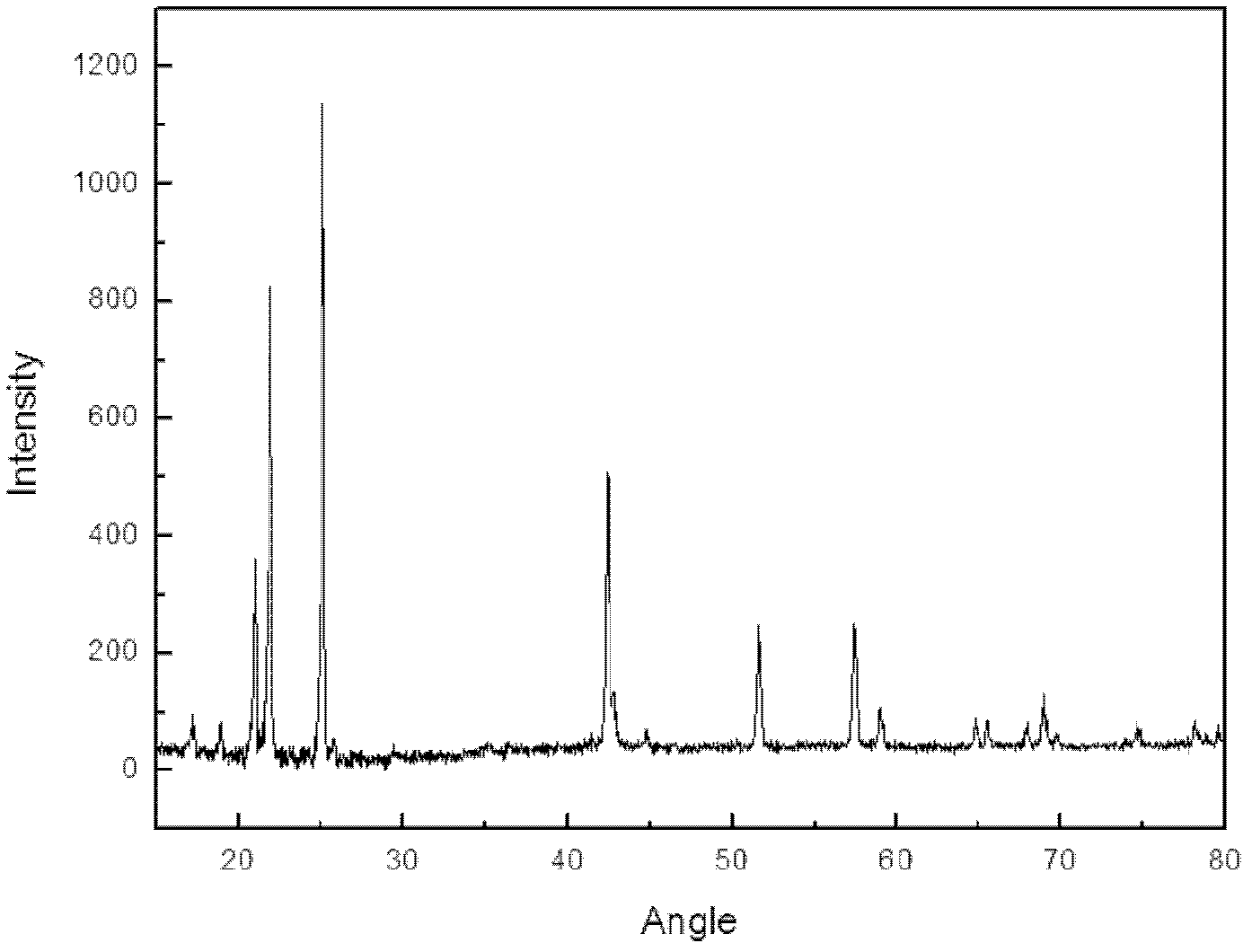
Preparation method of lithium hexafluorophate
A technology of lithium hexafluorophosphate and hexafluorophosphate, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of low yield and achieve the effects of environmental friendliness, mild reaction conditions and low corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Potassium hexafluorophosphate and lithium chloride with a molar ratio of 2.2:1 were dissolved in acetonitrile respectively to form solutions with a concentration of 10% and 20%; In the reaction kettle of the reactor, in dry air and under normal pressure, react at a temperature of 30°C for 8h; filter the above-mentioned feed liquid to remove insoluble matter, and evaporate the solution under reduced pressure at a temperature of 65°C to remove part of the solvent, and then dry In the air, cool and crystallize at a temperature of 10°C, filter, recrystallize, and recover the mother liquor to obtain the complex Li(CH 3 EN) 4 PF 6 , to obtain pure lithium hexafluorophosphate after vacuum drying. According to the alkali titration method, HF was not detected, and the moisture content measured by the Karl Fischer method was 15ppm. The impurity indicators detected by ICP spectroscopy met the requirements of HG / T 4066-2008, and the obtained lithium hexafluorophosphate had a puri...
Embodiment 2
[0036] Dissolve sodium hexafluorophosphate and lithium chloride with a molar ratio of 3:1 in ethylene glycol dimethyl ether and ethanol respectively to form solutions with a concentration of 30% and 25%; add the above two solutions with In a reactor with a stirrer, a thermometer and a reflux condenser, react at a temperature of 25°C for 2 hours under a nitrogen atmosphere and at normal pressure; filter the above-mentioned feed liquid to remove insoluble matter, and evaporate the solution under reduced pressure at a temperature of 65°C. Remove part of the solvent, cool and crystallize at -20°C under a nitrogen atmosphere, filter, recrystallize, and recover the mother liquor to obtain the complex LiPF of lithium hexafluorophosphate and ethylene glycol dimethyl ether 6 (C 4 h 10 o 2 ) 2 , to obtain pure lithium hexafluorophosphate after vacuum drying. According to the alkaline titration method, HF was not detected, and the moisture content measured by the Karl Fischer method ...
Embodiment 3
[0038]Dissolve ammonium hexafluorophosphate and lithium chloride with a molar ratio of 2.05:1 in acetone and acetonitrile respectively to form solutions with a concentration of 15% and 40%; and a reflux condenser reaction kettle, under a nitrogen atmosphere, in a normal pressure environment, react at a temperature of 0°C for 5.5h; filter the above-mentioned feed liquid to remove insoluble matter, and evaporate the solution under reduced pressure at a temperature of 20°C to remove part of the solvent , under a nitrogen atmosphere, cooled and crystallized at a temperature of -30°C, filtered, recrystallized, recovered the mother liquor, and obtained the complex Li(CH 3 EN) 4 PF 6 , to obtain pure lithium hexafluorophosphate after vacuum drying. According to the alkaline titration method, HF was not detected, and the moisture content was 16ppm according to the Karl Fischer method. The impurity indicators detected by ICP spectroscopy met the requirements of HG / T 4066-2008, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com