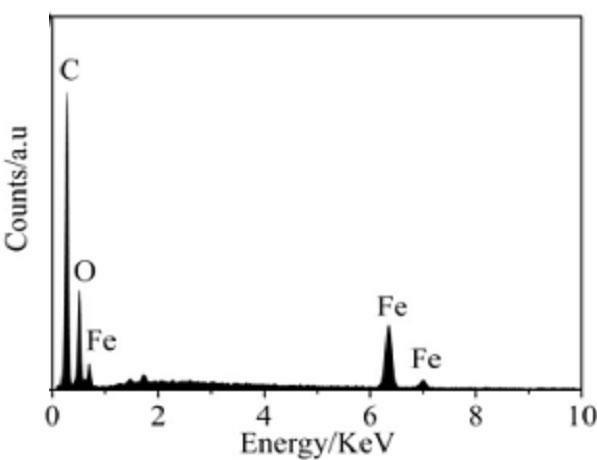
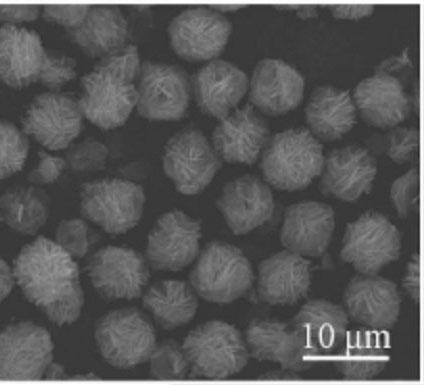
Magnetic ferric oxide micrometer flower material with multi-stage structure and preparation method thereof
A technology of ferric oxide and micron flowers is applied in the directions of iron oxide, iron oxide/iron hydroxide, etc., to achieve the effects of convenient practical industrial application, regular product morphology and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method for preparing a magnetic ferric oxide micron flower material with a multi-level structure, comprising the following steps:
[0029] (1) Take 0.005mol ferric chloride hexahydrate as iron source, 100ml ethylene glycol as solvent, add surfactant tetrabutylammonium chloride, stir under normal temperature and pressure, make ferric chloride hexahydrate and Surfactant dissolves completely, and obtaining ferric chloride concentration is the clear solution of 0.05 mol / L;
[0030] (2) Add 0.5mol urea to the clear solution prepared in step (1), stir, and mix thoroughly to obtain a reddish-brown clear solution;
[0031] (3) The above solution prepared in step (2) was reacted at 190°C for 60 min by the ethylene glycol-assisted method;
[0032] (4) Centrifuged after the reaction, the obtained yellow-green precipitate was washed 3 times with absolute ethanol, and then dried at 60°C for 12 h to obtain α-Fe 2 o 3 Precursor;
[0033] (5) Put the precursor in a vacuum atmosph...
Embodiment 2
[0041] (1) Take 0.01mol ferric chloride hexahydrate as the iron source, 100ml ethylene glycol as the solvent, then add 0.00065mol of surfactant tetrabutylammonium chloride, stir at normal temperature and pressure to make hexahydrate trichloride Iron and tensio-active agent dissolve completely, and obtaining ferric chloride concentration is the clear solution of 0.1 mol / L;
[0042] (2) Add 0.1mol urea to the clear solution prepared in step (1), stir, and mix thoroughly to obtain a reddish-brown clear solution;
[0043] (3) The above solution prepared in step (2) was reacted at 192°C for 70 min by ethylene glycol-assisted method;
[0044] (4) Centrifuged after the reaction, the obtained yellow-green precipitate was washed 3 times with absolute ethanol, and then dried at 65 °C for 12 h to obtain α-Fe 2 o 3 Precursor;
[0045] (5) Put the precursor in a vacuum atmosphere box-type furnace, introduce air, and calcine at a high temperature of 340-390 ° C for 5 hours to obtain the ...
Embodiment 3
[0047] (1) Take 0.06mol ferric chloride hexahydrate as iron source, 100ml ethylene glycol as solvent, then add surfactant cetyltrimethylammonium bromide, stir at normal temperature and pressure to make hexahydrate trimethylammonium bromide Ferric chloride and tensio-active agent dissolve completely, obtain the clarification solution that ferric chloride concentration is 0.6 mol / L;
[0048] (2) Add 0.03mol urea to the clear solution prepared in step (1), stir, and mix thoroughly to obtain a reddish-brown clear solution;
[0049] (3) The above solution prepared in step (2) was reacted at 197°C for 30 min by the ethylene glycol-assisted method;
[0050] (4) Centrifuged after the reaction, the obtained yellow-green precipitate was washed 4 times with absolute ethanol, and then dried at 55°C for 13 h to obtain α-Fe 2 o 3 Precursor;
[0051] (5) The precursor is placed in a vacuum atmosphere box furnace, air is introduced, and it is calcined at a high temperature of 380-460 °C fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com