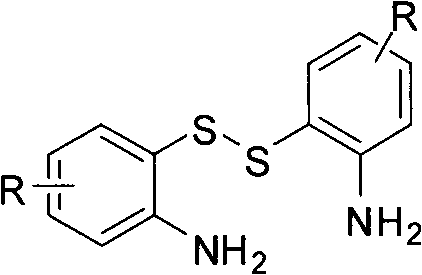
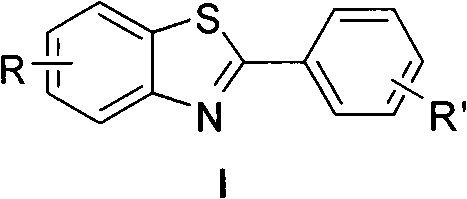
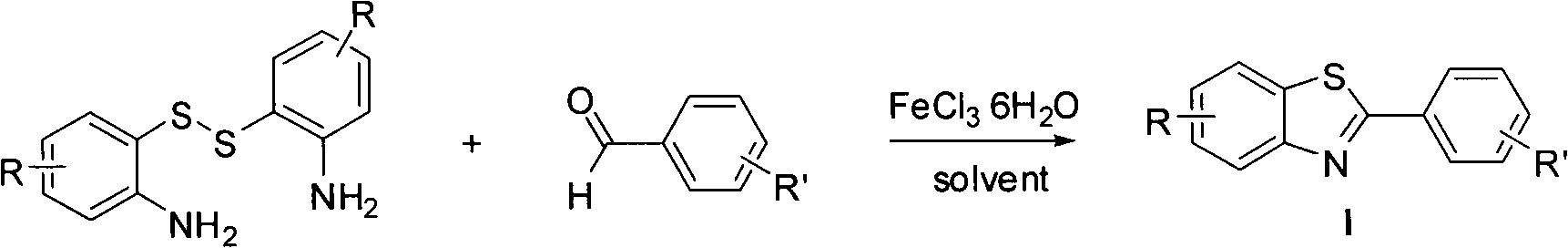
Method for synthesizing multi-substituted benzothiazole derivative
A technology of benzothiazole and synthesis method, which is applied in the field of medicine, can solve problems such as difficult industrial production, complex synthesis steps, and difficult operation, and achieve the effects of short reaction time, simple operation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1, Synthesis of 6-(3,-dimethylphenoxy)-2-phenylbenzothiazol-5-amine (R'=H in the structural formula)
[0017]
[0018] 1, Synthesis of 6-(3,5 dimethylphenoxy)-2-phenylbenzothiazol-5-amine
[0019] In the 25ml round bottom flask, add 0.10mmol raw material 6,6'-dithiobis(4-(3,5-dimethylphenoxy)benzene-1,3-diamine), 4ml methanol solvent, under stirring After dissolving the raw material, 0.02 mmol of ferric chloride hexahydrate was added, and stirred to dissolve. Finally, 0.12 mmol of benzaldehyde was added, and the mixture was stirred and reacted at room temperature for 10 min. It was found by TLC that the reaction of the disulfide raw material was complete, and the crude product was obtained after removing the solvent by using a rotary evaporator under reduced pressure. The crude product was separated by column chromatography (200-300 mesh silica gel) with petroleum ether and ethyl acetate or dichloromethane and ethyl acetate as eluent, and a white solid with ...
Embodiment 2
[0024] Example 2, Synthesis of 6-(3,5-dimethylphenoxy)-2-(4-bromophenyl)benzothiazol-5-amine (R'=Br in the structural formula)
[0025]
[0026] 1, Synthesis of 6-(3,5-dimethylphenoxy)-2-(4-bromophenyl)benzothiazol-5-amine
[0027] In the 25ml round bottom flask, add 0.10mmol raw material 6,6'-dithiobis(4-(3,5-dimethylphenoxy)benzene-1,3-diamine), 4ml methanol solvent, under stirring After dissolving the raw material, 0.02 mmol of ferric chloride hexahydrate was added, and stirred to dissolve. Finally, 0.12 mmol of p-bromobenzaldehyde was added, and the mixture was stirred and reacted at room temperature for 10 min. TLC showed that the reaction of the disulfide raw material was complete, and the crude product was obtained after removing the solvent by using a rotary evaporator under reduced pressure. The crude product was separated by column chromatography (200-300 mesh silica gel) using petroleum ether and ethyl acetate or dichloromethane and ethyl acetate as eluents, and...
Embodiment 3
[0032] Embodiment 3, the synthesis of 6-(3,-dimethylphenoxy)-2-(4-methoxyphenyl)benzothiazol-5-amine (R'=OCH in the structural formula 3 )
[0033]
[0034] 1. 6-(3,5-dimethylphenoxy)-2-(4-methoxyphenyl)benzothiazol-5-amine
[0035] In the 25ml round bottom flask, add 0.10mmol raw material 6,6'-dithiobis(4-(3,5-dimethylphenoxy)benzene-1,3-diamine), 4ml methanol solvent, under stirring After dissolving the raw material, 0.02 mmol of ferric chloride hexahydrate was added, and stirred to dissolve. Finally, 0.12 mmol of p-methoxybenzaldehyde was added, and the mixture was stirred and reacted at room temperature for 10 min. TLC detection revealed that the reaction of the disulfide raw material was complete, and the crude product was obtained after removing the solvent by using a rotary evaporator under reduced pressure. The crude product was separated by column chromatography (200-300 mesh silica gel) using petroleum ether and ethyl acetate or dichloromethane and ethyl acetate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com