Inorganic hydrous salt phase change energy storage microcapsule and preparation method thereof
An inorganic hydrated salt and phase change energy storage technology, which is applied in the direction of microcapsule preparation, microsphere preparation, energy storage, etc., can solve the problems of limiting the application of phase change energy storage materials, supercooled phase separation, etc., and overcome the phase separation phenomenon , reduce the degree of supercooling, good compactness effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
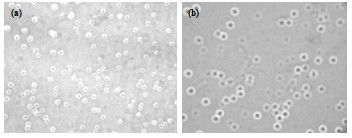
Examples
Embodiment 1
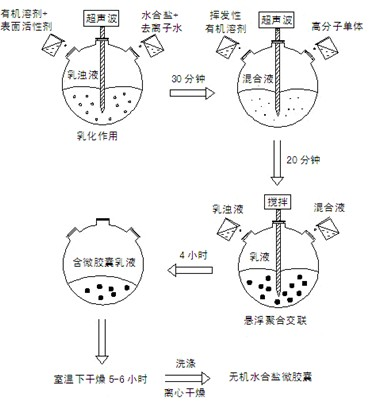
[0044] (1) Weigh 15g Na with weighing paper 2 HPO 4 12H 2 O and 4.5g of deionized water were placed in a 100ml beaker, and heated in a constant temperature water bath at 37°C to achieve the first step of dehydration until all crystals of disodium hydrogen phosphate dodecahydrate melted and became liquid. Subsequently, 0.375 g of Span80 and 50 ml of organic solvent toluene were added, mixed uniformly under stirring and placed in an ultrasonic cell pulverizer for physical dispersion for 30 minutes to form an emulsion.
[0045] (2) Measure 12.5ml methyl methacrylate, 2.25ml ethyl acrylate and other solutions, 0.003g sodium polyacrylate, 0.0072g stearic acid, 0.0036g dibenzoyl peroxide, mix with 50mL volatile organic solvent acetone in a 100ml beaker, and physically dispersed in an ultrasonic cell pulverizer for 20 minutes to form a mixed solution.
[0046] (3) Put the emulsion obtained in step (1) into a 250ml round-bottomed three-neck flask, place it in an intelligent tempera...
Embodiment 2
[0050] (1) Weigh 15g Na with weighing paper 2 HPO 4 12H 2 0 and 3g of deionized water are contained in a 100ml beaker, and heated in a constant temperature water bath at 40°C to realize the first step of dehydration until all crystals of disodium hydrogen phosphate dodecahydrate melt and become liquid. Then 0.1875g of Span80 and 45ml of organic solvent carbon tetrachloride were added, mixed evenly under stirring and placed in an ultrasonic cell pulverizer for physical dispersion for 40 minutes to form an emulsion.
[0051] (2) Measure 15ml of methyl methacrylate, 2.7ml of ethyl acrylate and other solutions, 0.00225g of sodium polyacrylate, 0.006g of stearic acid, 0.003g of dibenzoyl peroxide, and mix them with 55mL of volatile organic solvent chloroform 100ml beaker, and physically dispersed in an ultrasonic cell pulverizer for 30 minutes to form a mixed solution.
[0052](3) Put the emulsion obtained in step (1) into a 250ml round-bottomed three-neck flask, place it in an ...
Embodiment 3
[0056] (1) Weigh 15g Na with weighing paper 2 HPO 4 12H 2 0 and 4g of deionized water were placed in a 100ml beaker, and heated in a constant temperature water bath at 37°C to achieve the first step of dehydration until all crystals of disodium hydrogen phosphate dodecahydrate melted and became liquid. Subsequently, 0.3 g of Span80 and 50 ml of organic solvent toluene were added, mixed uniformly under stirring and placed in an ultrasonic cell pulverizer for physical dispersion for 20 minutes to form an emulsion.
[0057] (2) Measure 7.5ml methyl methacrylate, 1.35ml ethyl acrylate and other solutions, 0.0015g sodium polyacrylate, 0.0036g stearic acid, 0.003g dibenzoyl peroxide, mix with 50mL volatile organic solvent acetone in a 100ml beaker, and physically dispersed in an ultrasonic cell pulverizer for 20 minutes to form a mixed solution.
[0058] (3) Put the emulsion obtained in step (1) into a 250ml round-bottomed three-neck flask, place it in an intelligent temperature-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com