Gabapentin tablet and preparation method thereof
A gabapentin tablet, gabapentin technology, applied in pharmaceutical formulations, peptide/protein components, nervous system diseases, etc., can solve the problems of low gabapentin content, low drug content, and high equipment requirements, achieve good in vitro dissolution rate, and use less excipients , the effect of high drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Formula per 1000 tablets:
[0037] Gabapentin 800.0 g (80.00%)
[0038] Low-substituted hydroxypropyl cellulose 170.0g (17.00%)
[0039] Hypromellose 10.0g (1.00%)
[0040] Poloxamer 10.0g (1.00%)
[0041] Magnesium Stearate 10.0g (1.00%)
[0042] Preparation Process:
[0043] Take hydroxypropyl cellulose and dissolve it with ethanol to make a 10% solution; take another gabapentin and grind it to make the particle size meet the requirements (52% for particle size less than 80 μm, 82% for particle size less than 120 μm), and sieve through 80 mesh Mix loxamer and low-substituted hydroxypropyl cellulose evenly, add binder ethanol solution to make a moderate soft material, pass through 16 mesh, dry at 55°C, granulate, add appropriate amount of magnesium stearate, mix evenly, press piece.
[0044] test results:
[0045] 1. Friability: 0.07%.
[0046] 2. Lactam content: 0 day, ND (not detected); 40°C, RH (60%±10%) for 3 months, 0.076%.
Embodiment 2
[0048] Formula per 1000 tablets:
[0049] Gabapentin 800.0g (89.09%)
[0050] Low-substituted hydroxypropyl cellulose 71.3g (7.94%)
[0051] Hypromellose 8.9g (0.99%)
[0052] Poloxamer 8.9g (0.99%)
[0053] Magnesium Stearate 8.9g (0.99%)
[0054] Preparation Process:
[0055] Take hydroxypropyl cellulose and dissolve it with ethanol to make a 10% solution; take another gabapentin and grind it to make the particle size meet the requirements (61% for particle size less than 80 μm, 91% for particle size less than 120 μm), and sieve through 80 mesh Mix loxamer and low-substituted hydroxypropyl cellulose evenly, add binder ethanol solution to make a moderate soft material, pass through 16 mesh, dry at 55°C, granulate, add appropriate amount of magnesium stearate, mix evenly, press Tablets, coated.
[0056] test results:
[0057] 1. Friability of uncoated tablets: 0.18%.
[0058] 2. Lactam content: 0 days, ND; 40 ℃, RH (60% ± 10%) 3 months, 0.10%.
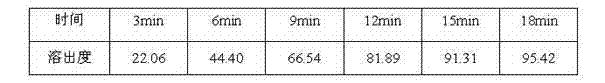
[0059] 3. Dissolution ...
Embodiment 3
[0062] Formula per 1000 tablets:
[0063] Gabapentin 800.0 g (80.00%)
[0064] Low-substituted hydroxypropyl cellulose 170.0g (17.00%)
[0065] Povidone K30 10.0g (1.00%)
[0066] Poloxamer 10.0g (1.00%)
[0067] Magnesium Stearate 10.0g (1.00%)
[0068] Preparation Process:
[0069] Take povidone K30, dissolve it with ethanol to make a solution of about 10%; take another gabapentin, grind it to make the particle size meet the requirements (52% for particle size less than 80 μm, 82% for particle size less than 120 μm), and sieve through 80 mesh Mix loxamer and low-substituted hydroxypropyl cellulose evenly, add binder ethanol solution to make a moderate soft material, pass through 16 mesh, dry at 55°C, granulate, add appropriate amount of magnesium stearate, mix evenly, press piece.
[0070] test results:
[0071] 1. Friability: 0.03%.
[0072] 2. Lactam content: 0 days, ND; 40 ℃, RH (60% ± 10%) 3 months, 0.082%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com