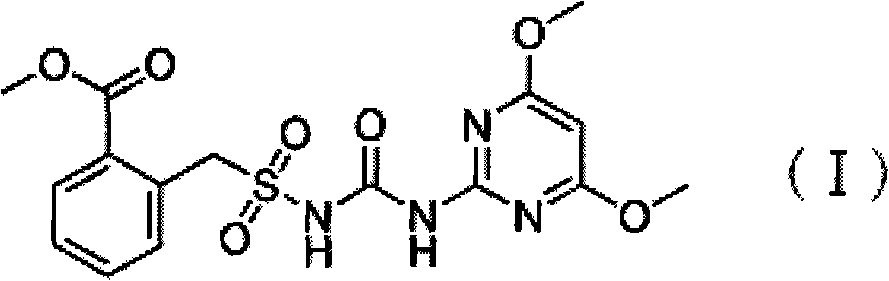
Preparation method of bensulfuron
A technology of bensulfuron-methyl and methyl benzylsulfonamide, which is applied in the field of preparation of bensulfuron-methyl, can solve the problems of low production safety, high product cost, and high cost, and achieve high production safety and small amount of three wastes produced , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In a 500 ml four-necked flask equipped with a stirrer, a thermometer, a reflux condenser and a constant pressure dropping funnel, add 45.8 grams of methyl o-formate benzylsulfonamide, 31.65 grams of 2-amino-4,6-dimethoxy Base pyrimidine, 200 ml of dry acetone solvent and 3.67 g of dry 4-dimethylaminopyridine, stir to cool down to 5 °C, dropwise add 32.55 g of ethyl chloroformate, slowly warm up to 15 °C, stir for 3.0 h, filter, wash, Dry to obtain 64.5 grams of white powder, the measured content of bensulfuron-methyl is 96.4%, and the calculated yield is 78.6%.
Embodiment 2
[0019] In a 500 ml four-necked flask equipped with a stirrer, a thermometer, a reflux condenser and a constant pressure dropping funnel, add 45.8 grams of methyl o-formate benzylsulfonamide, 31.65 grams of 2-amino-4,6-dimethoxy base pyrimidine, 200 ml of dry tetrahydrofuran solvent and 3.67 g of dry 4-dimethylaminopyridine, stirred and cooled to 5 °C, added dropwise 34.72 g of ethyl chloroformate, slowly raised to 20 °C, stirred for 3.0 h, filtered, washed, Dry to obtain 64.78 grams of white powder, the measured content of bensulfuron-methyl is 96.7%, and the calculated yield is 79.0%.
Embodiment 3
[0021] In a 500 ml four-neck flask equipped with a stirrer, a thermometer, a reflux condenser and a constant pressure dropping funnel, add 45.8 grams of methyl o-formate benzylsulfonamide, 31.65 grams of 2-amino-4,6-dimethoxy Base pyrimidine, 200 ml of dry pyridine solvent and 3.67 g of dry pyridine, stir and cool down to 5 °C, dropwise add 32.55 g of ethyl chloroformate, slowly raise the temperature to 25 °C, stir for 3.0 h, filter, wash, and dry to obtain a white powder 65.28 gram, the measured content of bensulfuron-methyl was 97.2%, and its yield was calculated to be 79.61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com