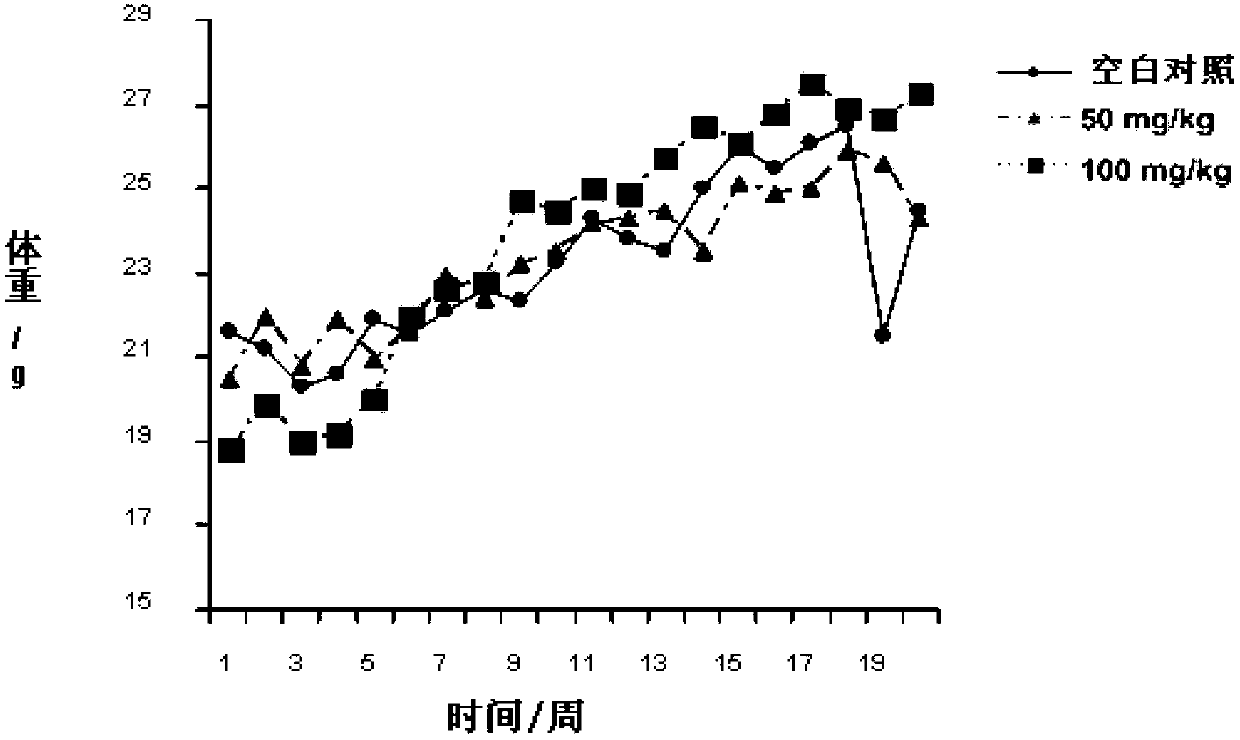
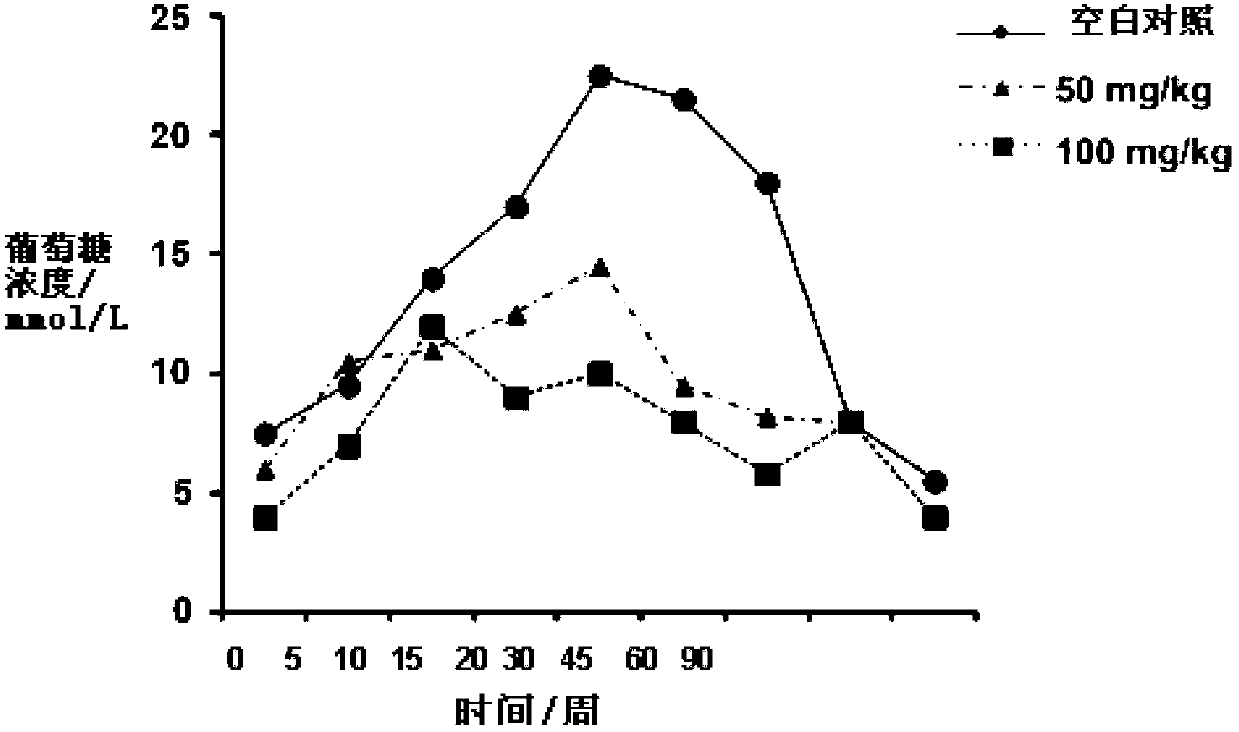
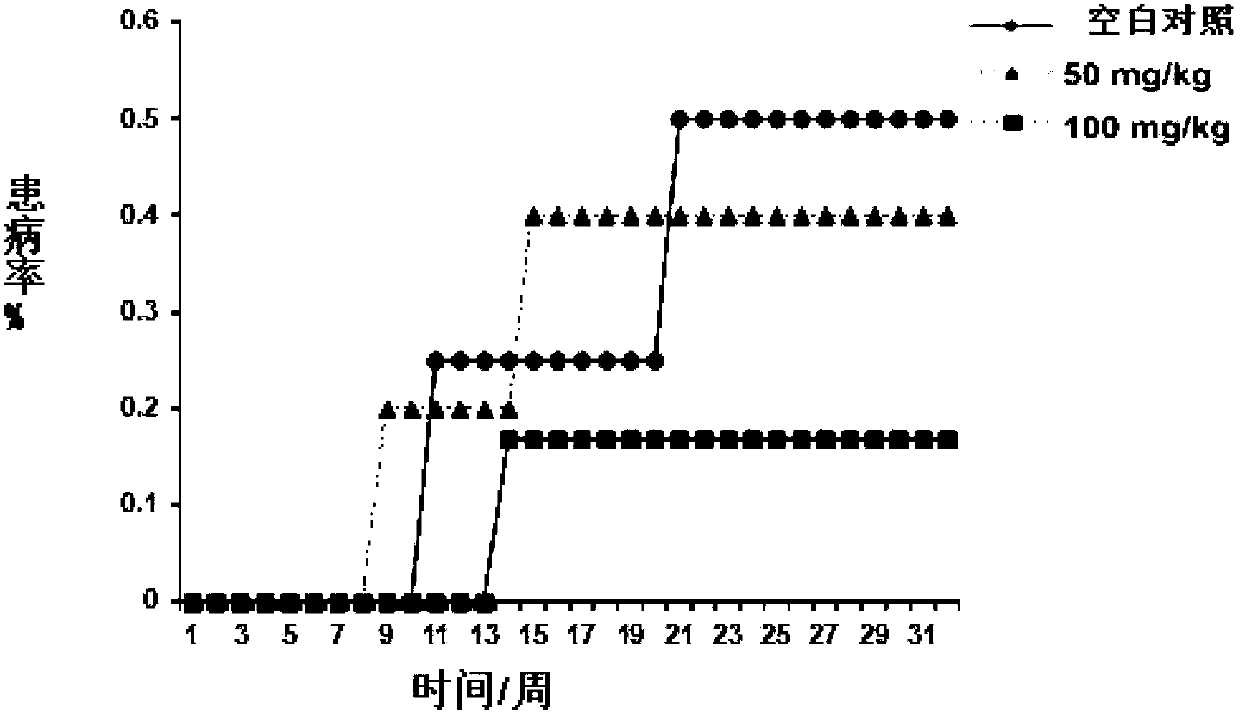
Compound for preventing or treating autoimmune diabetes and preparation method and application thereof
An autoimmune and compound technology, applied in the direction of steroids, allergic diseases, medical preparations containing active ingredients, etc., to achieve the effect of preventing or treating autoimmune diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0037] Example 12 - Preparation of Diosgenin Acetoxybenzoate (Compound 2):
[0038] (1) Preparation of 2-acetoxybenzoyl chloride:
[0039]
[0040] In a 50ml reaction vial, add aspirin (4.50g, 25mmol), 2 drops of N,N'-dimethylformamide (DMF) and 3ml of thionyl chloride SOCl 2 (36mmol), quickly connect the spherical condenser (calcium chloride drying tube is installed on the top), and import the tail gas into the sodium hydroxide solution for absorption. Slowly heat up to 70°C under stirring, and keep this temperature constant until no bubbles emerge from the tail gas end. Remove excess thionyl chloride by suction filtration under reduced pressure to obtain light yellow oily liquid, namely 2-acetoxybenzoyl chloride. (TLC: chloroform:methanol=9:1, R f =0.83); Dissolve 2-acetoxybenzoyl chloride in tetrahydrofuran THF to make 25ml of liquid, and seal it for later use.
[0041] (2) Preparation of diosgenin 2-acetoxybenzoate:
[0042]
[0043] Into a 150ml three-necked fl...
Embodiment 2
[0044] Example 2 Preparation of N-(2-acetoxybenzoyl)-glycine diosgenin ester (compound 3)
[0045] (1) Preparation of 2-acetoxybenzoyl chloride:
[0046]
[0047]In a 50ml reaction bottle, add 4.50g (25mmol) of aspirin, 2 drops of N,N'-dimethylformamide DMF and 3ml of thionyl chloride SOCl 2 (36mmol), quickly connect the spherical condenser (calcium chloride drying tube is installed on the top), and import the tail gas into the sodium hydroxide solution for absorption. Slowly heat up to 70°C under stirring, and keep this temperature constant until no bubbles emerge from the tail gas end. Excess thionyl chloride was removed by suction filtration under reduced pressure to obtain 2-acetoxybenzoyl chloride as an oily liquid. (Chloroform:methanol=9:1, Rf=0.83; dissolve 2-acetoxybenzoyl chloride in tetrahydrofuran THF to make 25ml of liquid, sealed for later use)
[0048] (2) Preparation of N-tert-butoxycarbonylglycine diosgenin ester
[0049]
[0050] In a 50ml reaction b...
Embodiment 32
[0057] Example 32- Preparation of (4-isobutylphenyl) diosgenin propionate (compound 4):
[0058]
[0059] 1.03g (5mmol) of ibuprofen, 2.07g (5mmol) of diosgenin, 1g of 4A molecular sieve, 122mg (1mmol) of 4-dimethylaminopyridine DMAP, 172mg (0.2mmol) of p-toluenesulfonic acid TsOH, 100ml of anhydrous dichloromethane, add dropwise N,N'-dicyclohexylcarbodiimide DCC 1.55g (7.5mmol, dissolved in 50ml of anhydrous dichloromethane) under stirring at room temperature, and check the progress of the reaction by TLC until the reaction is no longer Go, filter. After the filtrate was washed several times with 10g / L citric acid solution and saturated sodium chloride solution successively, it was washed with anhydrous sodium sulfate Na 2 SO 4 Dry and separate on a silica gel column to obtain diosgenin 2-(4-isobutylphenyl)propionate as a white solid, 2.14 g. (Petroleum ether: ethyl acetate=4:1, Rf=0.68) 1H NMR (400MHz, CDCl3) δ7.20(d, J=7.8Hz, 2H), 7.08(d, J=7.7Hz, 2H), 5.34 (dd,J=15....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com