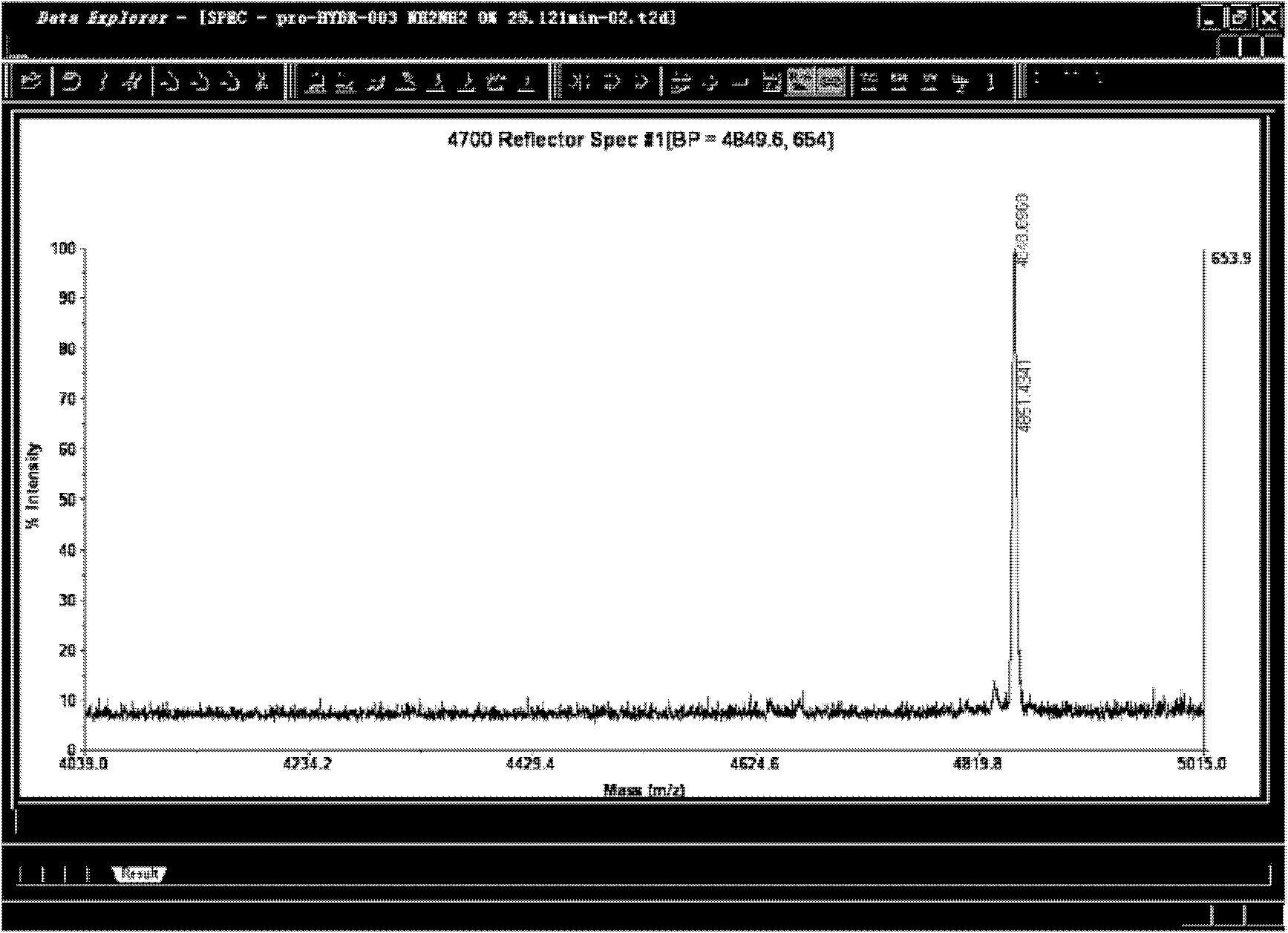
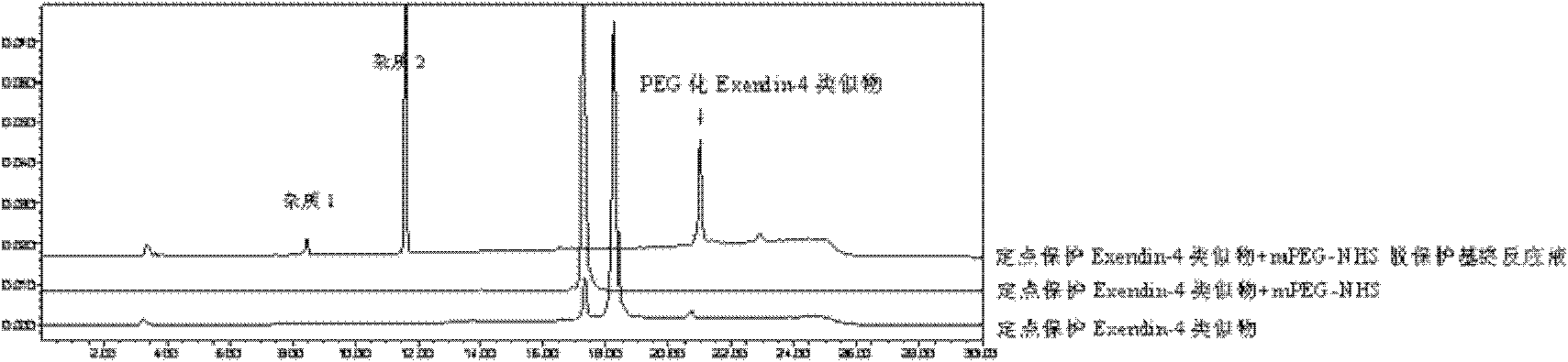
Fixed point mono-substituted pegylation of Exendin analogue and preparation method thereof
A technology of PEGylation and PEGylation, which is applied in the field of fixed-point monosubstituted PEGylation of Exendin analogues, can solve the problems of easy oxidation and shedding of cysteine, and unclear effects of drug efficacy and toxicity and other problems to achieve the effect of avoiding multiple substitution reactions and reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) Amino acid monomers used in the synthesis
[0065] Fmoc-His(Trt)-OH, Dde-His(Trt)-OH, Fmoc-Ala-OH, Fmoc-Gly-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Thr(tBu)-OH, Fmoc- Phe-OH, Fmoc-Ser(tBu)-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Leu-OH, Fmoc-Lys(Boc)-OH, Fmoc-Lys(Dde)-OH, Fmoc-Gln( Trt)-OH, Fmoc-Val-OH, Fmoc-ILe-OH, Fmoc-Trp(Boc)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH
[0066] Abbreviations above are: Fmoc 9-fluorenylmethoxycarbonyl; Boc tert-butoxycarbonyl; Trt trityl; OtBu tert-butyloxy; tBu tert-butyl; Dde N-[1-(4,4-dimethyl base-2,6-dioxocyclohexylidene)
[0067] (2) Reagents: N,N-diisopropylethylamine, diisopropylcarbodiimide (DIC), N,N-dimethylformamide (DMF), dichloromethane, hexahydropyridine, 1 -Hydroxybenzotriazole, Rink Amide resin, ninhydrin, methanol, triisopropylsilane, trifluoroacetic acid.
[0068] (3) Operation
[0069] A Synthesis: Take 0.25 mmol scale as an example, weigh 0.5 g of Rink Amide resin into a reactor, weigh 1 mmol of amino acids, activate by...
Embodiment 2
[0087] Method for site-directed protection of Exendin-4 analogs PEGylation
[0088] In this example, conventional amino PEGylation modification reagents such as (SC-PEG, SS-PEG, NHS-PEG, etc.) are used to protect the only free side chain on the Exendin-4 analog that can be PEGylated. Amino group for binding modification. Among them, the PEGylation reagent is preferably 40KD Y-type NHS-PEG, and the site-directed protection Exendin-4 analog is preferably
[0089] (Fmoc)His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-(Dde)Lys-Gln-Z-Glu-Glu-Glu-Ala-Val-Lys-Leu-Phe- Ile-Glu-Trp-Leu-(Dde)Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-(Dde)Lys or
[0090] (Fmoc)His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-(Dde)Lys-Gln-Z-Glu-Glu-Glu-Ala-Val-(Dde)Lys-Leu -Phe-Ile-Glu-Trp-Leu-(Dde)Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-Lys.
[0091] Take 2 g of site-protected Exendin-4 analog and 26 g of 40KD Y-type NHS-PEG (about 1.5:1 molar ratio to the polypeptide) and dissolve it in...
Embodiment 3
[0094] Separation and purification method of PEGylated Exendin-4 analogs
[0095] The final reaction solution of PEGylation of exendin-4 analogs with site-specific protection is respectively subjected to reverse-phase HPLC, ion exchange, ultrafiltration concentration, molecular sieves and freeze-drying to obtain pure PEGylated Exendin-4 analog raw materials.
[0096] A. SOURCE reversed-phase HPLC purification
[0097] Mobile phase: A phase A 20mM HAc 5% acetonitrile; B phase 20mM HAc 50% acetonitrile
[0098] Column: GE Fineline Pilot 35
[0099] Filler: SOURCE 30RPC 175mL
[0100] Flow rate: 30mL / min
[0101] Elution gradient: Equilibrate 2 column volumes after loading, 0-30% 5min, 30%-100% 5min
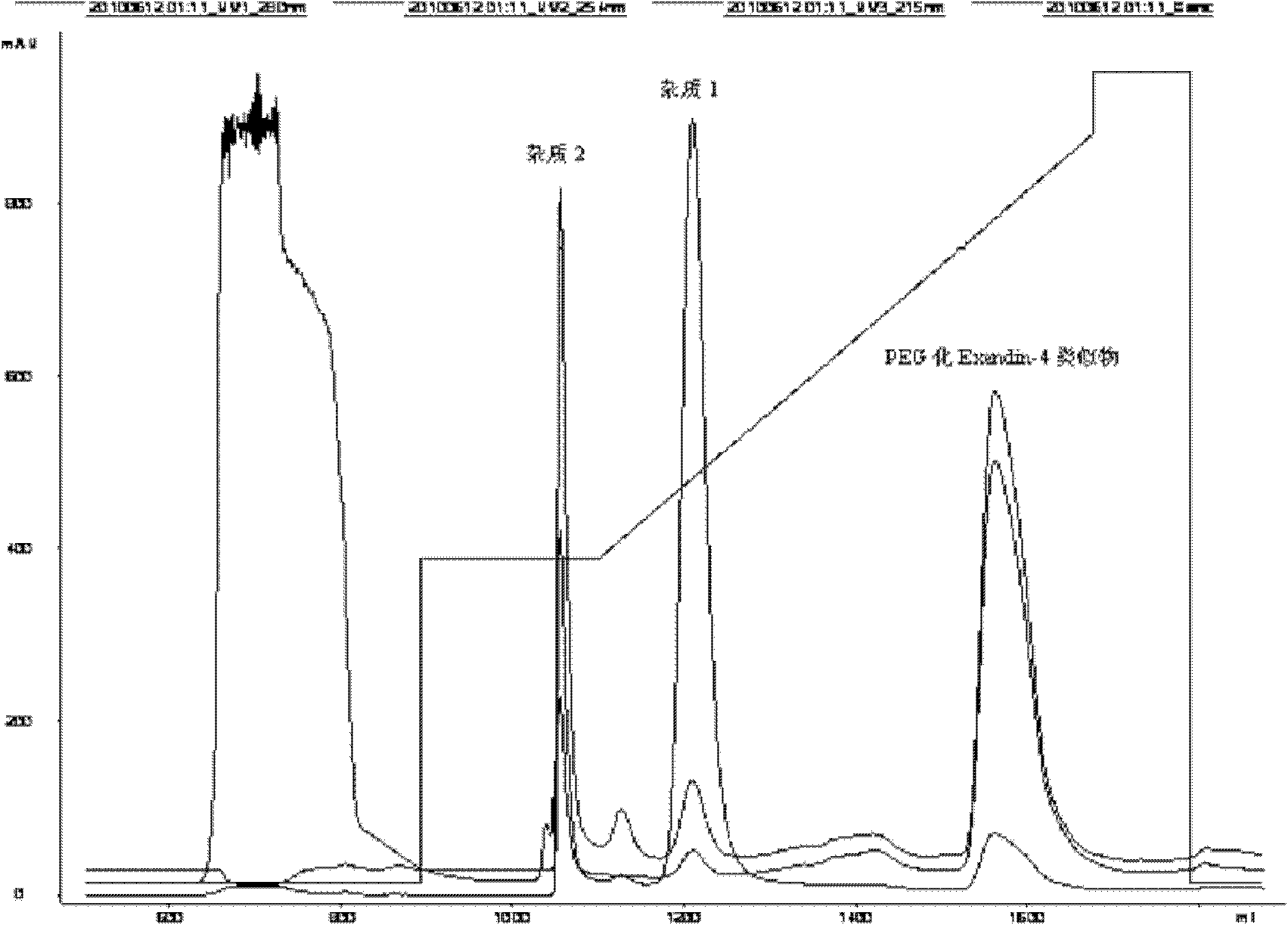
[0102] image 3 is the SOURCE purification chromatogram of the PEGylated Exendin-4 analog.
[0103] B, SP cation exchange purification
[0104] Mobile phase: Phase A 10mM pH3.5CBS
[0105] Phase B1 10mM pH3.5 CBS+0.15M NaCl
[0106] Phase B2 10mM pH3.5 CBS+1.5M NaCl
[010...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com