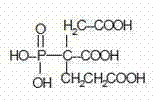
Batch method for producing 2-phosphonobutane-1, 2, 4-tricarboxylic acid and byproduct methyl chloride
A technology for butane tricarboxylic acid and by-product methyl chloride, applied in the chemical industry, can solve the problems of poor scale inhibition performance and slow release performance, difficult to control reaction stability, poor operation safety, etc., and achieves good environmental protection and improved Worker working environment, high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
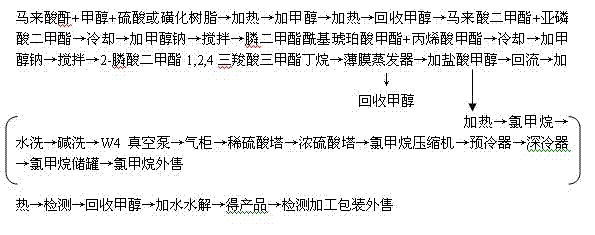
[0034] Put 500kg of methanol into the reaction kettle and start stirring, open the manhole cover, pour 1000kg of maleic anhydride and 5kg of sulfuric acid into the kettle, turn on the heating, add 700kg of methanol dropwise, the temperature is 115°C ~ 130°C, heat at 132°C to recover methanol, and obtain maleic acid. Acid dimethyl ester;
[0035] Put 500kg of dimethyl maleate and 363kg of dimethyl phosphite into the reaction kettle, turn on the cooling water, add 20kg of sodium methoxide catalyst dropwise, at a temperature of 10°C to 65°C, and stir within 8 hours to synthesize phosphine dimethyl acyl succinate Acid methyl ester;
[0036] Continue to add 290kg of methyl acrylate, add sodium methoxide alkaline catalyst dropwise, and stir at a temperature of 10°C to 65°C within 8 hours to synthesize 2-dimethyl phosphonate-1,2,4tricarboxylic acid trimethylbutane;
[0037] Pour the solution containing dimethyl 2-phosphonate-1,2,4 tricarboxylate butane into the thin film evaporator,...
Embodiment 2
[0043] Put 500kg of dimethyl maleate and 365kg of dimethyl phosphite into the reaction kettle, turn on the cooling water, add 20kg of potassium methylate catalyst dropwise, the temperature is 50-55°C, and stir within 8 hours to synthesize phosphinedimethyl acyl succinic acid Methyl ester;
[0044] Continue to add 300kg of methyl acrylate, add sodium methoxide alkaline catalyst dropwise, and stir at a temperature of 50-55°C within 8 hours to synthesize 2-dimethyl phosphonate-1,2,4 tricarboxylic acid trimethylbutane;
[0045] Pour the solution containing dimethyl 2-phosphonate-1,2,4 tricarboxylate butane into the thin film evaporator, turn on the heating, the temperature is less than 110°C, and recover methanol;
[0046] Pour all the dimethyl 2-phosphonate-1,2,4 tricarboxylate butane at the bottom of the thin film evaporator into the acidolysis reaction kettle, add 1200kg of methanol hydrochloride, heat to reflux at a temperature of 75°C to 110°C, and open the Vacuum pump, reco...
Embodiment 3
[0050] Put 500kg of dimethyl maleate and 370kg of dimethyl phosphite into the reaction kettle, turn on the cooling water, add 20kg of potassium methylate catalyst dropwise, the temperature is 50-55°C, and stir within 8 hours to synthesize phosphinedimethyl acyl succinic acid Methyl ester;
[0051] Continue to feed 350kg of methyl acrylate, dropwise add potassium methoxide basic catalyst, temperature 50-55°C, stir within 8 hours to synthesize 2-dimethyl phosphonate-1,2,4 tricarboxylic acid trimethyl butane;
[0052] Pour the solution containing dimethyl 2-phosphonate-1,2,4 tricarboxylate butane into the thin film evaporator, turn on the heating, the temperature is less than 110°C, and recover methanol;
[0053] Pour all the dimethyl 2-phosphonate-1,2,4 tricarboxylate butane at the bottom of the thin film evaporator into the acidolysis reaction kettle, add 1300kg of methanol hydrochloride, heat to reflux at a temperature of 75°C to 110°C, and open the Vacuum pump, recovery of m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com