Method for synthesizing poly(N-vinylcaprolactam)/polycaprolactone segmented copolymer
A vinyl caprolactam and block copolymer technology, applied in the field of chemical synthesis, can solve problems such as poor solubility, and achieve the effect of improving properties and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Poly N-vinyl caprolactam / polycaprolactone (PNVCL- b -PCL1) Synthesis of block copolymers
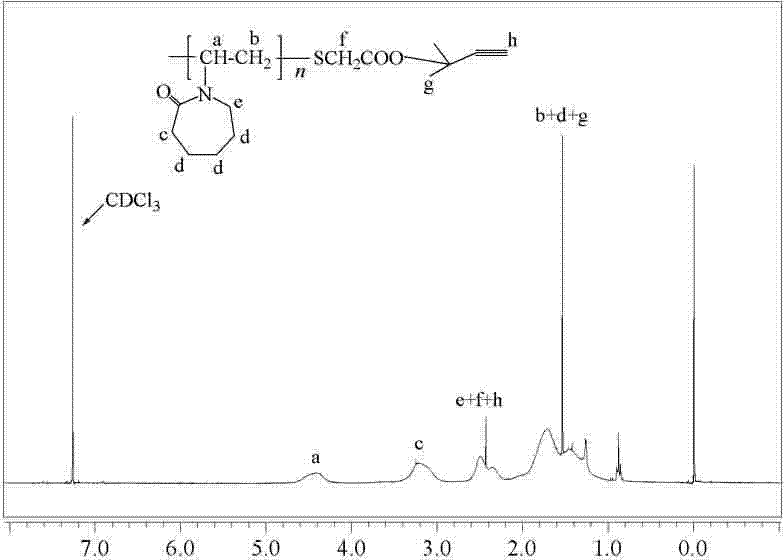
[0030] (1) Alkyne-terminated poly-N-vinyl caprolactam (PNVCL-C≡CH)
[0031] 1. Synthesis of carboxyl-terminated poly-N-vinylcaprolactam (PNVCL-COOH)
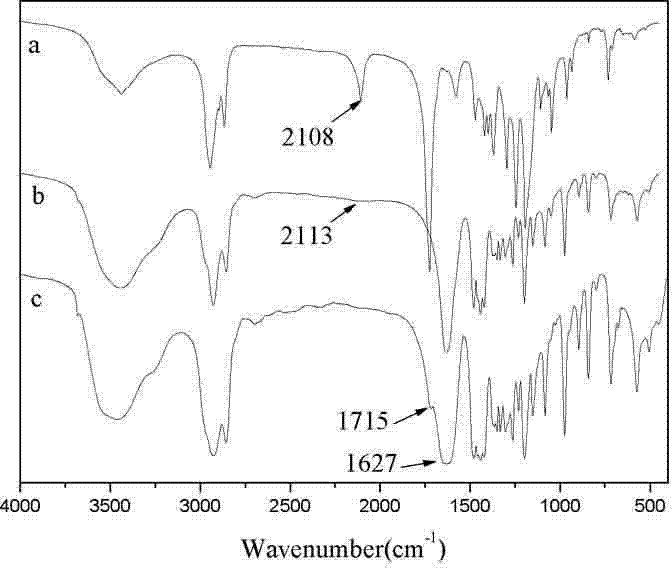
[0032] Weigh 22.78g (0.16mol) of N-vinylcaprolactam (NVCL), 0.36g (2.20mmol) of azobisisobutyronitrile (AIBN) and dissolve in 80mL of dioxane, and stir for 30min at room temperature under nitrogen. The temperature was raised to 68°C, and the nitrogen gas flow was continued for 30 minutes. Weigh 0.60g (6.52mmol) of thioglycolic acid (TGA), dissolve it in 20mL of dioxane, then add it into the above reaction flask, and react with nitrogen gas for 12h. After the reaction was finished, dioxane was removed by rotary evaporation to obtain a crude product. The crude product was dissolved in 100 mL of dichloromethane and poured into 700 mL of n-hexane for precipitation. The above operation was repeated 4 times, and the obtai...
Embodiment 2
[0054] Example 2 Poly N-vinyl caprolactam / polycaprolactone (PNVCL- b -PCL2) Synthesis of block copolymers
[0055] (1) Synthesis of alkyne-terminated poly-N-vinyl caprolactam (PNVCL-C≡CH): same as Example 1.
[0056] (2) Synthesis of azido-terminated polycaprolactone (N 3 -PCL 2 )
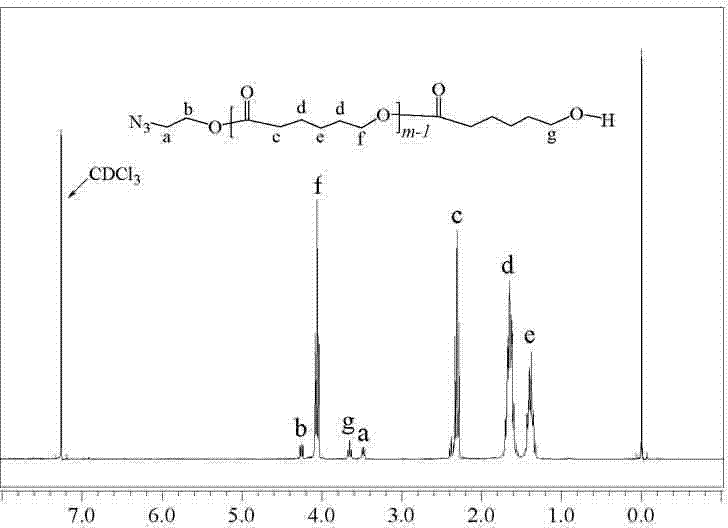
[0057] Weigh 5.13g (45mmol) ε-caprolactone (ε-CL), 0.13g (1.49mmol) azide ethanol (N 3 -CH 2 CH 2 OH), 0.15g (0.37mmol) stannous octoate (Sn(Oct) 2 ) was dissolved in 15mL of toluene, vacuumed and blown with nitrogen for 6 times, and stirred at 80°C for 12h. The toluene was removed by rotary evaporation to obtain a colorless viscous liquid. The viscous liquid was dissolved with 10mL of dichloromethane and poured into 80mL of n-hexane for precipitation. Repeat the above operation 3 times, and the precipitate is vacuum-dried to obtain a white powder end azido polycaprolactone (N 3 -PCL 2 ) 5.10g, yield 94%.
[0058] (3) Synthesis of PNVCL- b -PCL2
[0059] Weigh 1.10g (0.27mmol) PNVCL...
Embodiment 3
[0060] Example 3 Poly N-vinyl caprolactam / polycaprolactone (PNVCL- b -PCL3) Synthesis of block copolymers
[0061] (1) Synthesis of alkyne-terminated poly-N-vinyl caprolactam (PNVCL-C≡CH): same as Example 1.
[0062] (2) Synthesis of azido-terminated polycaprolactone (N 3 -PCL 3 )
[0063] Weigh 3.77g (33.07mmol) ε-caprolactone (ε-CL), 0.055g (6.32mmol) azidoethanol (N 3 -CH 2 CH 2 OH), 0.050g (0.12mmol) stannous octoate (Sn(Oct) 2 ) was dissolved in 15mL of toluene, vacuumed and blown with nitrogen for 6 times, and stirred at 80°C for 12h. The toluene was removed by rotary evaporation to obtain a colorless viscous liquid. The viscous liquid was dissolved with 10mL of dichloromethane and poured into 80mL of n-hexane for precipitation. Repeat the above operation 3 times, and the precipitate is vacuum-dried to obtain 1.43 g of white powder, which is PNVCL- b -PCL3 Yield 88%. The product was confirmed by infrared spectroscopy, nuclear magnetic resonance and gel per...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com