Heat-resistant beta-mannanase and its coding gene, recombinant bacterium and use
A mannanase and recombinant bacteria technology, applied in the application, genetic engineering, plant genetic improvement and other directions, can solve the problems of poor heat resistance, inability to resist the temperature of feed pelleting, limiting the use of β-mannanase, etc. Good acid stability, wide catalytic pH range and high specific activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1, Discovery of thermostable β-mannanase (MANN-cx1 protein) and its coding gene
[0038] 1. Error-prone PCR amplification of β-mannanase gene
[0039] Using the optimized wild-type mannanase gene as a template, the error-prone PCR reaction system contains: dNTP (2.5mM), dCTP (10mM), dTTP (10mM), Mg 2+ (25mM), Mn 2+ (10 mM), Taq DNA polymerase (5 U). Primers were E: 5′-CCG GAA TTC TTG CCA AAG GC-3' (the underline is the restriction endonuclease EcoR I site), X: 5'-GC T CTA GA T TAA GCA GAA TC-3' (the underline is the restriction endonuclease XbaI cutting site). The PCR reaction conditions were: denaturation at 94°C for 1 min, annealing at 52°C for 30 s, extension at 72°C for 1 min, and 30 cycles. PCR products were detected by 1% agarose gel electrophoresis.
[0040] 2. Construction and screening of mutant expression library
[0041] The error-prone PCR product was digested with restriction endonucleases EcoR I and Xba I, and then connected to the self-r...
Embodiment 2
[0046] Embodiment 2, the construction of recombinant bacteria and the expression of thermostable β-mannanase (MANN-cx1 protein)
[0047] 1. Construction of engineering bacteria
[0048] 1. Synthesize the DNA (MANN-cx1 gene) shown in sequence 2 of the sequence listing.
[0049] 2. Using the MANN-cx1 gene synthesized in step 1 as a template, perform PCR amplification with a primer pair composed of F1 and R1 to obtain a PCR amplification product.
[0050] F1: 5'-CCG GAA TTC TTG CCA AAG GC-3' (underlined EcoR I restriction recognition sequence);
[0051] R1: 5'-GC T CTA GA T TAA GCA GAA TC-3' (Xba I restriction recognition sequence is underlined).
[0052] 3. The PCR amplified product of step 2 was double-digested with restriction enzymes EcoR I and Xba I, and the digested product was recovered.
[0053] 4. Digest the vector pPICZαA with restriction endonucleases EcoR I and Xba I to recover the vector backbone (about 3600 bp).
[0054] 5. Ligate the digested product of st...
Embodiment 3
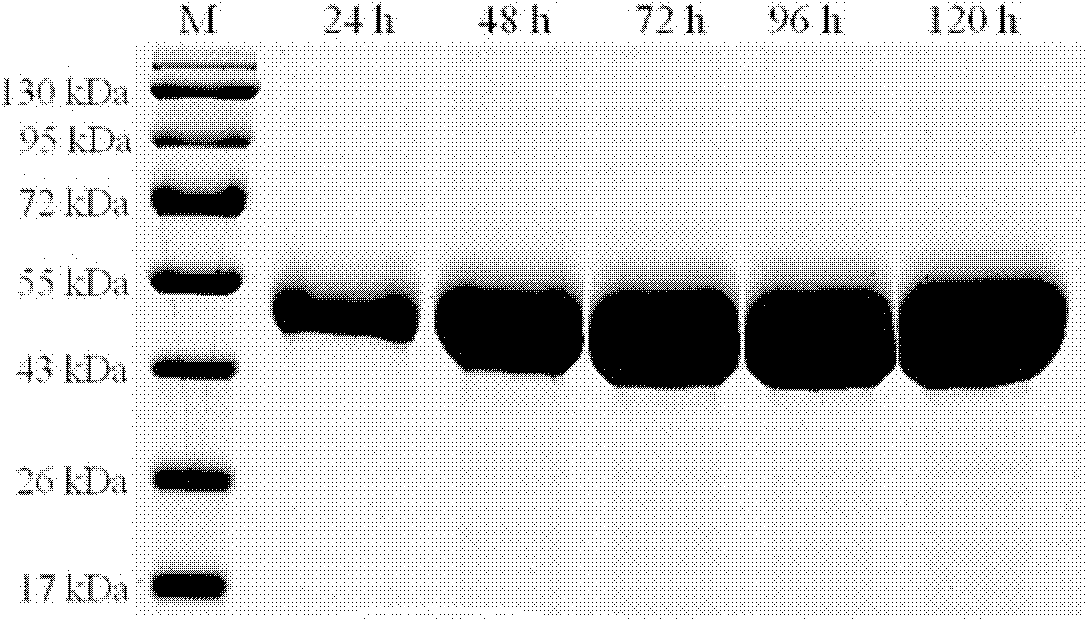
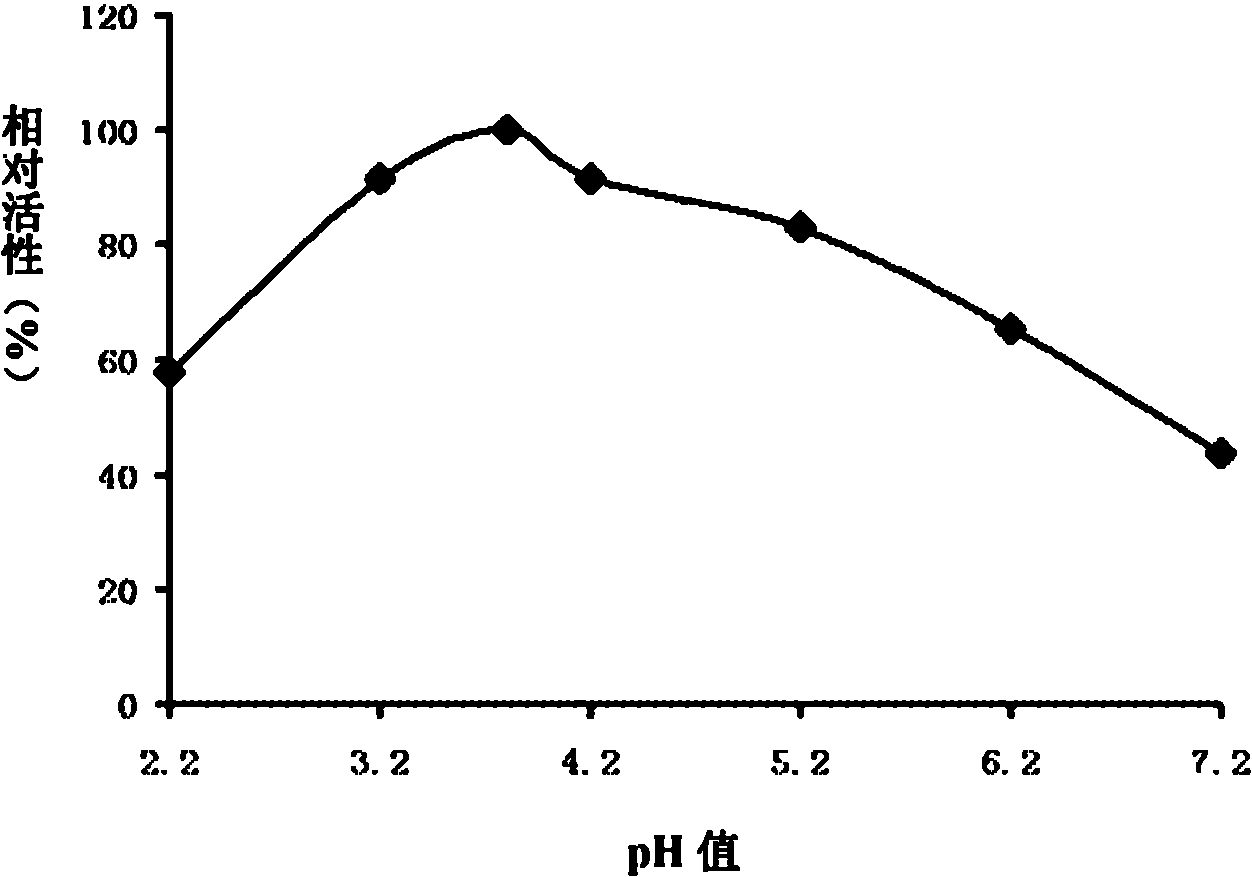
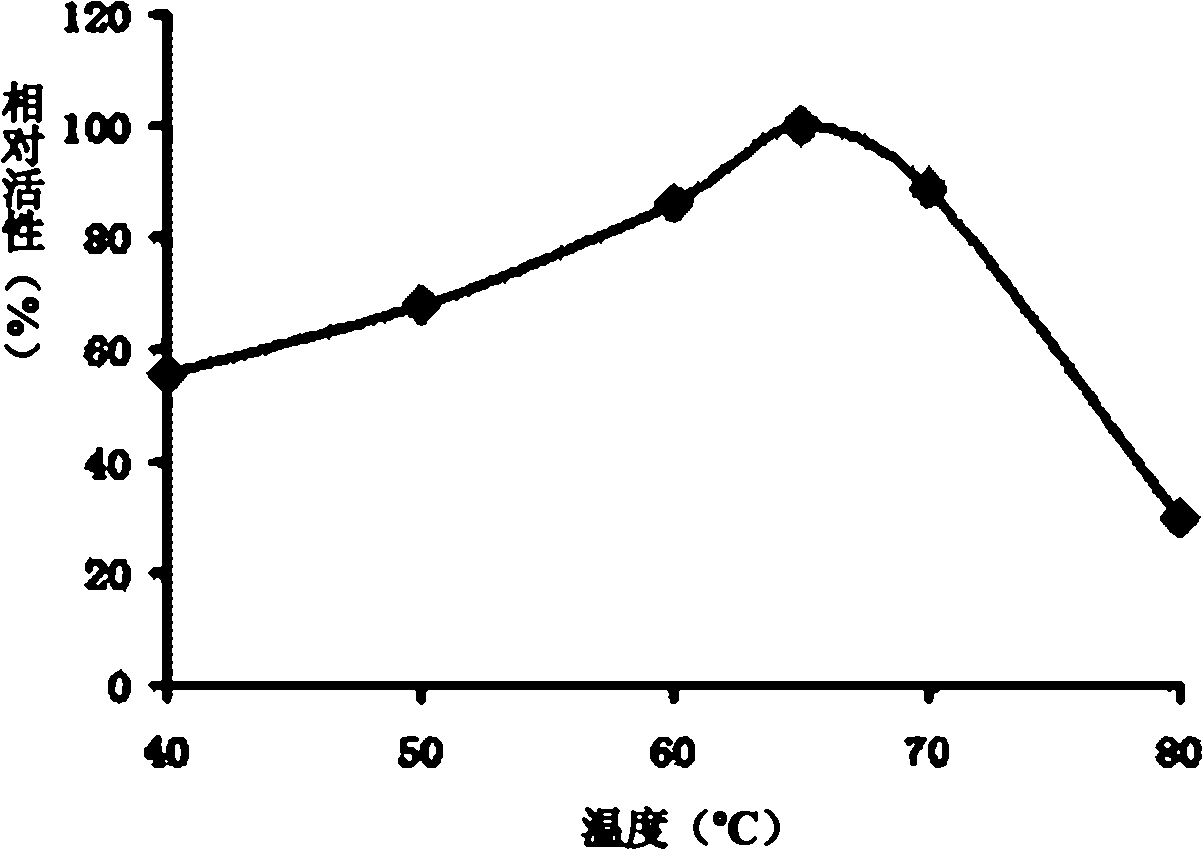
[0083] Example 3, Enzymatic property analysis of thermostable β-mannanase (MANN-cx1 protein)
[0084] 1. Determination of the optimum pH value
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com