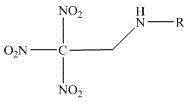
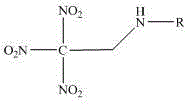
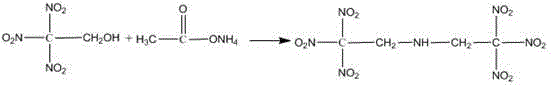
Trinitroethyl energetic compound and preparation method thereof
A technology of trinitroethyl function and trinitroethyl, which is applied in the field of preparation of trinitroethyl energetic compounds, can solve the problems of high sensitivity, explosion hazard, explosion, etc., and meet environmental requirements, thermal Good stability and high oxygen content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of trinitromethane, the specific method is as follows:
[0029] For ice bath reaction, a 500 mL four-necked flask was equipped with mechanical stirring, a condenser, and a thermometer, and 180 mL of concentrated sulfuric acid was added to the four-necked flask, and 22.4 g (0.2 mol) of 4,6-dihydroxy-pyrimidine was added in batches, mechanically Stir until completely dissolved. Slowly add 64 g (1 mol) of fuming nitric acid dropwise, and control the dropping rate so that the reaction temperature does not exceed 10 °C. After the dropwise addition, continue to react for 20 min, heat up to 10°C in a water bath, stir for 10 min, then raise the temperature to 20°C, stir for 30 min, and finally raise the temperature to 40°C, stir for 90 min, pour into 800 g of ice-water mixture after the reaction , stirred for 60 min, extracted the aqueous solution with dichloromethane three times (200 mL each time), combined the dichloromethane phases, dried with anhydrous magnesium ...
Embodiment 2
[0031] Synthesis of trinitromethane, the specific method is as follows:
[0032] For ice bath reaction, a 500 mL four-necked flask was equipped with mechanical stirring, a condenser, and a thermometer, and 150 mL of concentrated sulfuric acid was added to the four-necked flask, and 22.4 g (0.2 mol) of 4,6-dihydroxy-pyrimidine was added in batches, mechanically Stir until completely dissolved. Slowly add 51 g (0.8 mol) of fuming nitric acid dropwise, and control the dropping rate so that the reaction temperature does not exceed 10 °C. After the dropwise addition, continue to react for 20 min, heat up to 10°C in a water bath, stir for 10 min, then raise the temperature to 20°C, stir for 30 min, and finally raise the temperature to 40°C, stir for 90 min, pour into 800 g of ice-water mixture after the reaction , stirred for 60 min, extracted the aqueous solution with dichloromethane three times (200 mL each time), combined the dichloromethane phases, dried with anhydrous magnesium ...
Embodiment 3
[0034] Synthetic trinitroethanol, concrete method is as follows:
[0035] A 1000 mL four-neck flask was equipped with mechanical stirring, a condenser, and a thermometer, and 350 mL of carbon tetrachloride was added to it, and a dichloromethane solution of trinitromethane (containing 7.5 g of trinitromethane, 49.6 mmol) and polymer Formaldehyde 1.62 g (mass fraction 95%, containing 49.6 mmol formaldehyde), heated to 62 °C with mechanical stirring for 3 h, then heated to 75 °C and refluxed for 30 min. After the reaction solution was filtered, the filtrate was rotary evaporated to 100 mL under reduced pressure, and placed in a refrigerator to precipitate long needle-like crystals with a melting point of 72 °C and a yield of 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com