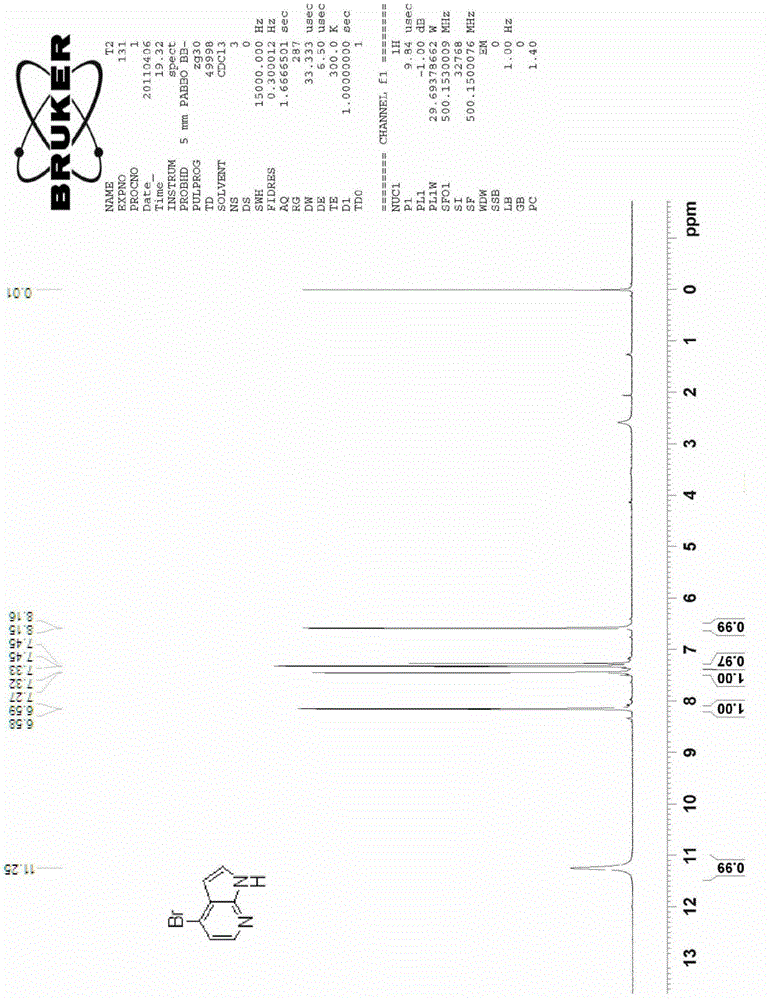
Preparation method for 4-substituted-7-azaindole
A technology of azaindole and organic solvent, applied in the field of synthesis of 4-substituted-7-azaindole, which can solve the problems of difficult industrialization, high price, and difficult removal, and achieve low cost, cheap price, and easy removal Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis of embodiment 1N-oxidation-7-azaindole
[0034]
[0035] 7-Azaindole (12.6 g, 0.102 mol) was added to tetrahydrofuran (120 ml), while the reaction was cooled to 5 ° C with an ice bath, hydrogen peroxide (6.1 g, 0.122 mol) was added dropwise to the reaction with stirring In the system, the temperature was slowly raised to room temperature, and reacted for 3 hours. The reaction solvent was spun to 30 ml on a rotary evaporator, and 60 ml of n-hexane was added thereto, and an off-white solid was precipitated. Filter and wash the filter cake with n-hexane, and dry the filter cake to obtain 12.8 g (0.0954 mol) of the target product, with a yield of 93.6%.
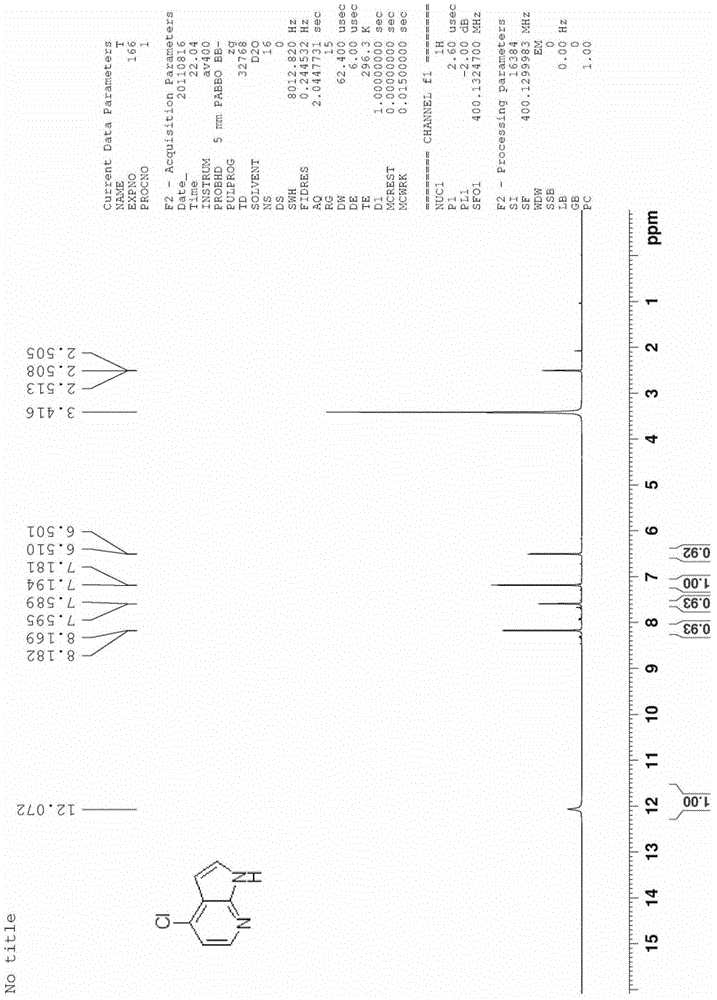
[0036] The synthesis of embodiment 24-chloro-7-azaindole
[0037]
[0038] N-oxide-7-azaindole (5.8 g, 0.043 mol) was added to acetonitrile (60 ml), and POCl was added under room temperature and stirring 3 (32.7 g, 0.215 mol). The reactant was heated to 80°C-100°C and reacted for 30 minutes, then di...
Embodiment 44
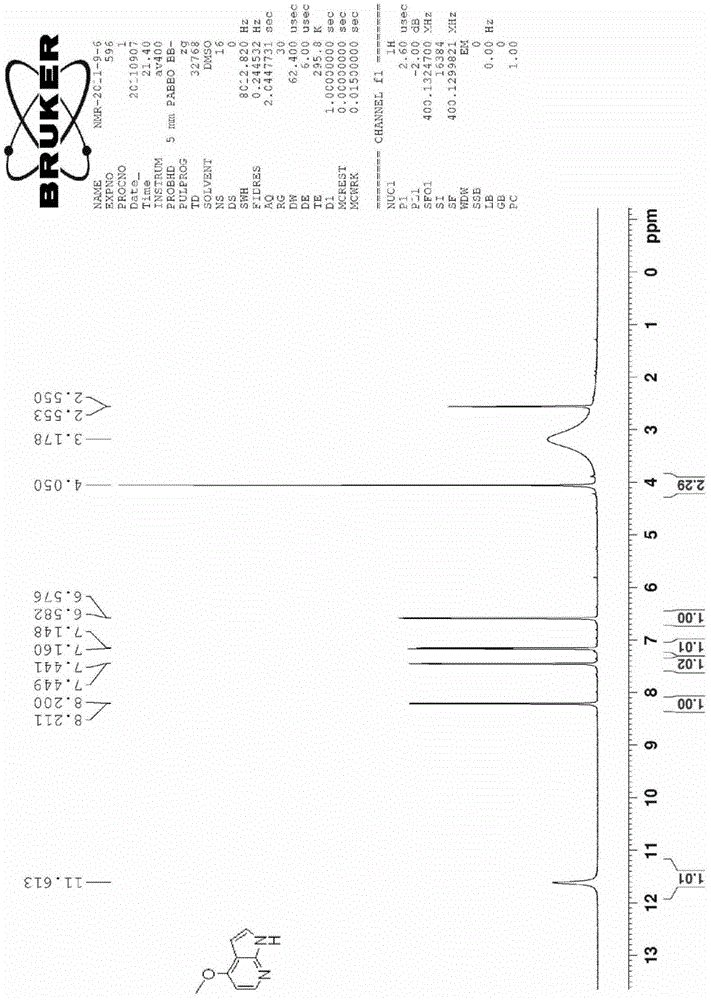
[0042] The synthesis of embodiment 44-methoxy group 7-azaindole
[0043]
[0044] 4-Bromo-7-azaindole (3.8 g, 0.019 mol) and sodium methoxide (1.3 g, 0.024 mol) were added to DMF (50 ml) at room temperature. The reaction temperature was raised to 110°C-130°C and reacted for 8 hours, then cooled to room temperature. DMF was removed by distillation under reduced pressure, and 30 ml of water was added to the residue. Extracted 3 times with 20ml of ethyl acetate, combined the organic phases, dried, and spin-dried to give a light yellow solid, which was recrystallized from methanol or ethanol to give 4-methoxy 7-azaindole (CAS 122379-63-9) 1.6 g, NMR spectrum as image 3 Shown, yield is 57.2%. The overall yield was 42.4%.
Embodiment 4
[0046] Other compounds were synthesized using the products of Examples 2-4 as raw materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com