Purification method of cefotiam hydrochloride and aseptic powder injection of cefotiam hydrochloride
A technology of cefotiam hydrochloride and purification method, which is applied in antibacterial drugs, organic chemistry, powder transportation, etc., can solve the problems of cefotiam hydrochloride acetone exceeding the standard, acetone solvent exceeding the standard, etc., and achieve the solution of acetone exceeding the standard, low organic residue, The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
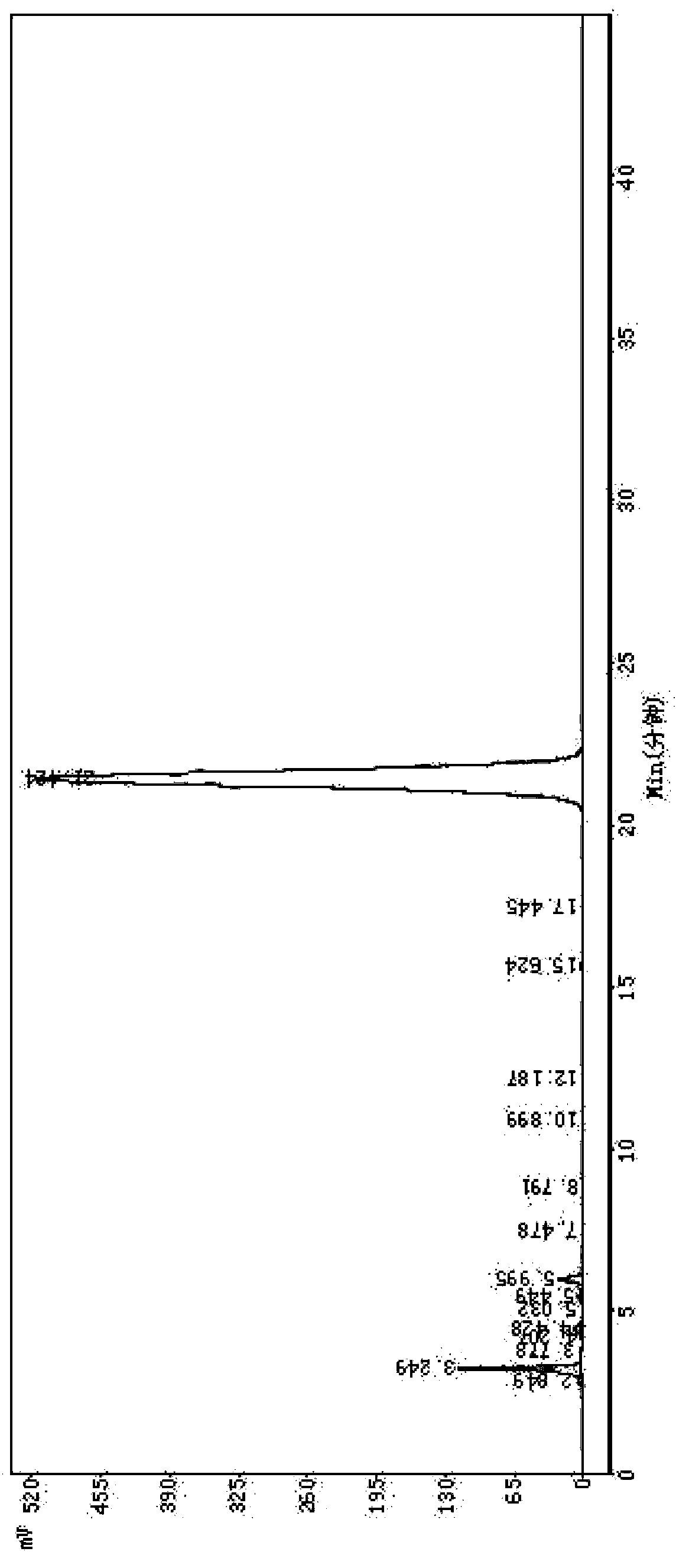
[0051] Example 1 Purification of Cefotiam Hydrochloride Crude Product, Preparation of Cefotiam Hydrochloride Sterile Powder Injection
[0052] Weigh 500g of crude cefotiam hydrochloride with a purity of 92.1% (which contains impurities 7-ACA, DMMT, 7-ATA-HACA, and 7-DMT) and add it to the reaction kettle, add 1L of water to dissolve it, and then add 2.0g of activated carbon for injection , heated to 50°C, and filtered after 30 minutes; after filtration, take the obtained filtrate, add 3L of acetone, heat and stir to dissolve, and use a 0.22μm microporous membrane to filter under positive pressure at a temperature below 0.1Mpa; freeze the filtrate to 0°C, and Low-temperature crystallization for 2 hours, after the crystallization is completed, filter; put the obtained crystals into a crystallization tank, add 1.5L of methyl tert-butyl ether for beating and washing, and suction filter to obtain white crystals; then pour water into the white crystals Saturated with nitrogen, after...
Embodiment 2
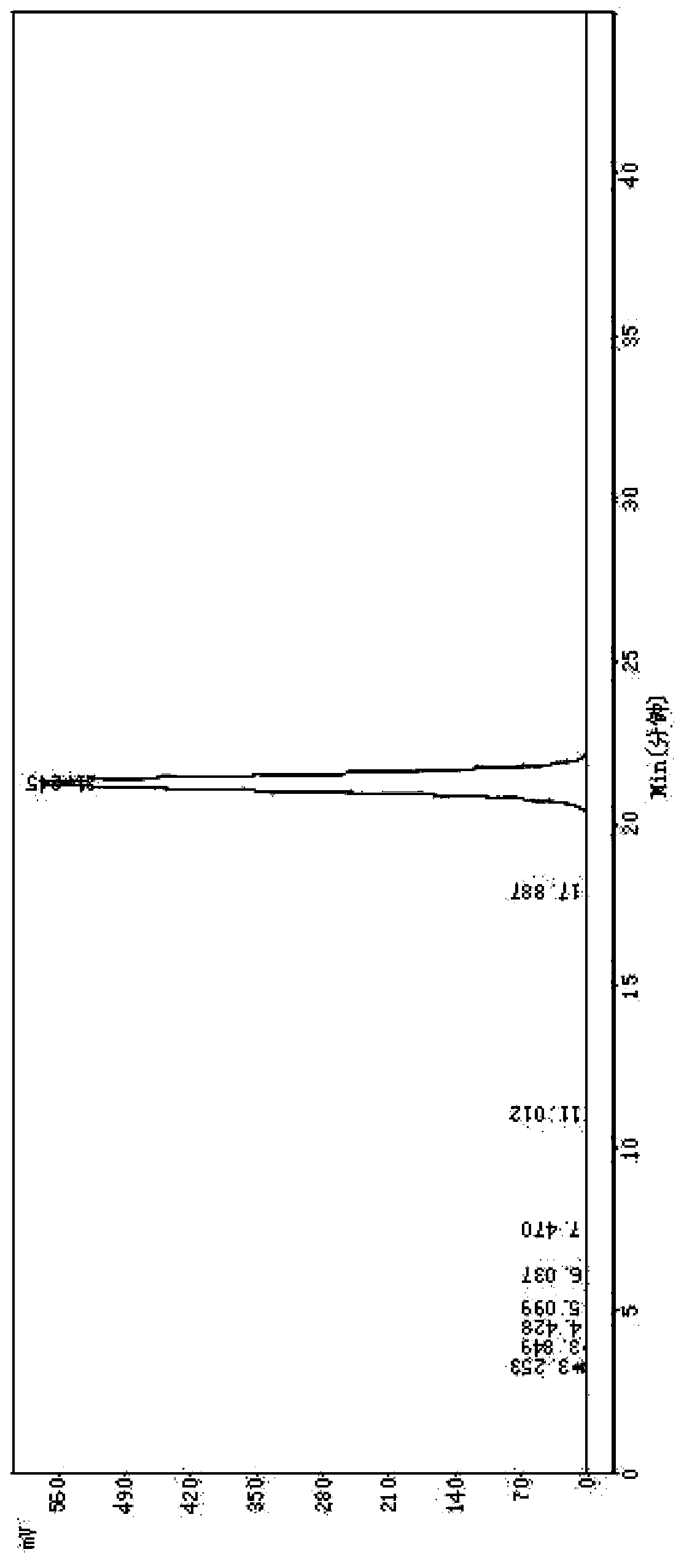
[0060] Example 2 Purification of Cefotiam Hydrochloride Crude Product, Preparation of Cefotiam Hydrochloride Sterile Powder Injection
[0061] Weigh 500g of crude cefotiam hydrochloride with a purity of 92.1% (which contains impurities 7-ACA, DMMT, 7-ATA-HACA, and 7-DMT) and add it to the reaction kettle, add 0.5L of water to dissolve, add 3.0g for injection Activated carbon, heated to 60°C, and filtered after 30 minutes; take the obtained filtrate, add 2L acetone, heat and stir to dissolve, and use a 0.22μm microporous filter membrane to filter under positive pressure below 0.1Mpa; freeze the filtrate to 0°C, and cool Crystallize for 2 hours, after the crystallization is completed, filter; put the obtained crystals into a crystallization tank, add 2L of ether for beating and washing, and filter with suction to obtain white crystals; then pass water-saturated nitrogen into the white crystals, and filter with suction for 5 hours , vacuum-dried at 25° C. for 3 hours; the crystal...
Embodiment 3
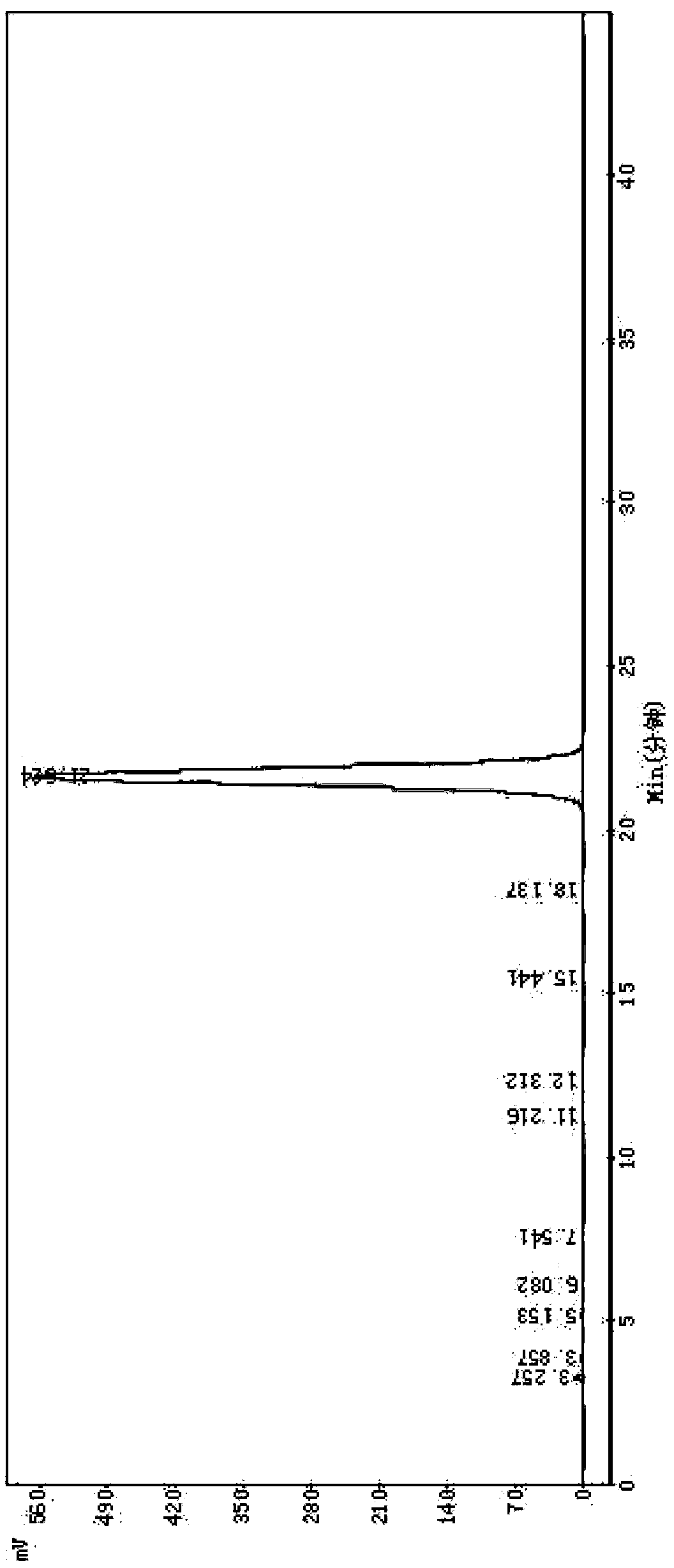
[0067] Example 3 Purification of Cefotiam Hydrochloride Crude Product, Preparation of Cefotiam Hydrochloride Sterile Powder Injection
[0068] Weigh 500g of crude cefotiam hydrochloride with a purity of 92.1% (which contains impurities 7-ACA, DMMT, 7-ATA-HACA, and 7-DMT) and add it to the reaction kettle, add 0.5L of water to dissolve, add 5.0g for injection Activated carbon, heated to 55°C, and filtered after 30 minutes; take the obtained filtrate, add 2L acetone, heat and stir to dissolve, and use a 0.22μm microporous filter membrane to filter under positive pressure at a temperature below 0.1Mpa; freeze the filtrate to 0°C, and refrigerate Crystallize for 2 hours, after the crystallization is completed, filter; put the obtained crystals into a crystallization tank, add 1.5 L of methyl tert-butyl ether for beating and washing, and filter with suction to obtain white crystals; then pour water into the white crystals to saturate Nitrogen, after suction filtration for 4 hours, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com