Chemical nickel plating solution and preparation method thereof, method for carrying out nickel plating on nano-LiFePO4/C composite material by using chemical nickel plating solution, and resulting product thereof
A technology of electroless nickel plating solution and composite materials, applied in the direction of coating, etc., can solve the problems of small tap density, etc., and achieve the effect of high tap density, low internal resistance and excellent discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
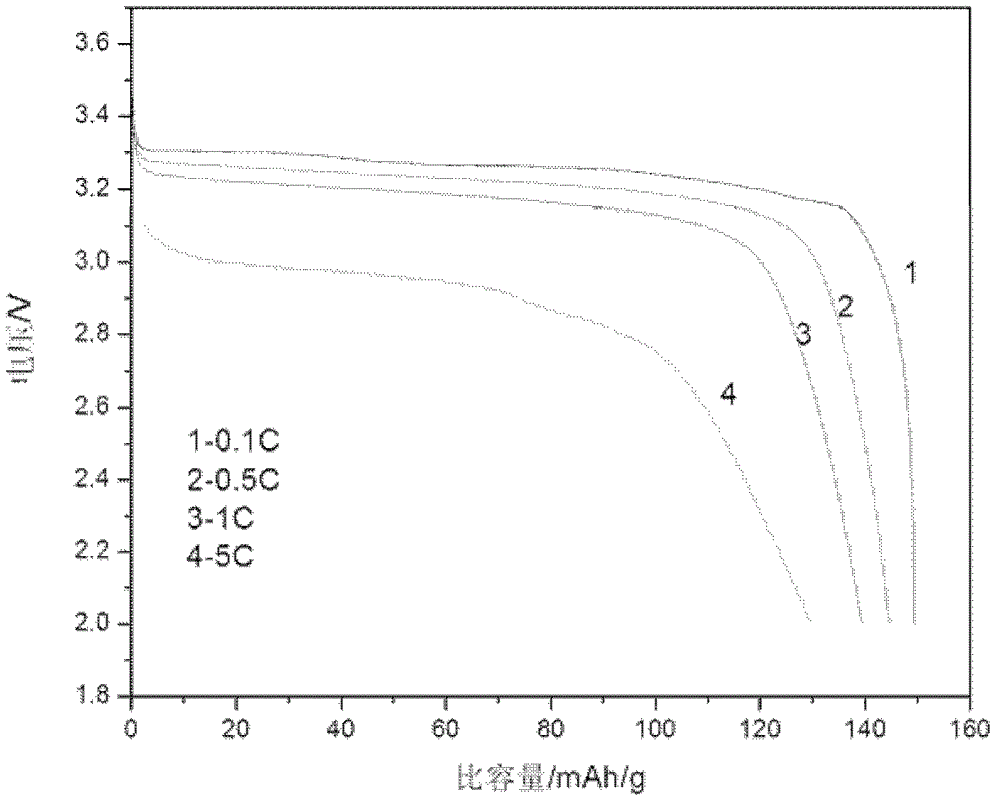
Embodiment 1
[0032] 1. Composition of electroless nickel plating solution
[0033] Nickel sulfate 33g / L;
[0034] Sodium hypophosphite 28g / L;
[0035] Sodium acetate 10g / L;
[0036] Citric acid 20g / L;
[0037] Alanine 10g / L;
[0038] Ammonia to adjust the pH to 4.6.
[0039] Second, the preparation of electroless nickel plating solution
[0040] 1) Prepare citric acid solution and sodium acetate solution respectively, and mix the two solutions to obtain mixed solution A;
[0041] 2) preparing a nickel sulfate solution, adding the mixed solution A to the nickel sulfate solution to obtain a mixed solution B;
[0042] 3) Prepare sodium hypophosphite solution and alanine solution respectively, and mix the two solutions to obtain mixed solution C;
[0043] 4) Add mixed solution C to mixed solution B, add distilled water to make up to 1 L to obtain mixed solution D, adjust the pH of mixed solution D to 4.6 with ammonia water, and obtain an electroless nickel plating solution.
[0044] 3....
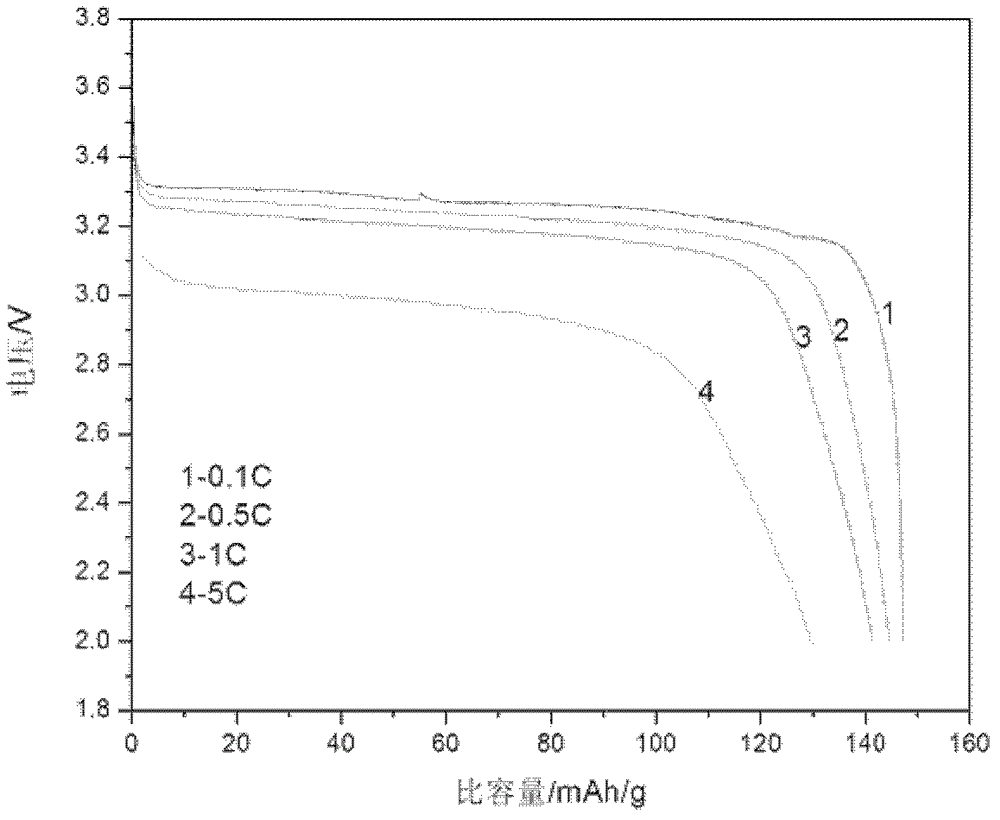
Embodiment 2
[0060] 1. The composition of electroless nickel plating solution:
[0061] Nickel sulfate 30g / L;
[0062] Sodium hypophosphite 26g / L;
[0063] Sodium acetate 15g / L;
[0064] Citric acid 25g / L;
[0065] Cysteine 20g / L;
[0066] Ammonia to adjust the pH to 5.2.
[0067] Second, the preparation of electroless nickel plating solution:
[0068] 1) Prepare citric acid solution and sodium acetate solution respectively, and mix the two solutions to obtain mixed solution A;
[0069] 2) preparing a nickel sulfate solution, adding the mixed solution A to the nickel sulfate solution to obtain a mixed solution B;
[0070] 3) Prepare sodium hypophosphite solution and cysteine solution respectively, and mix the two solutions to obtain mixed solution C;
[0071] 4) Add the mixed solution C to the mixed solution B, add distilled water to make the volume up to 1 L to obtain the mixed solution D, adjust the pH of the mixed solution D to 5.2 with ammonia water, and obtain the electrole...
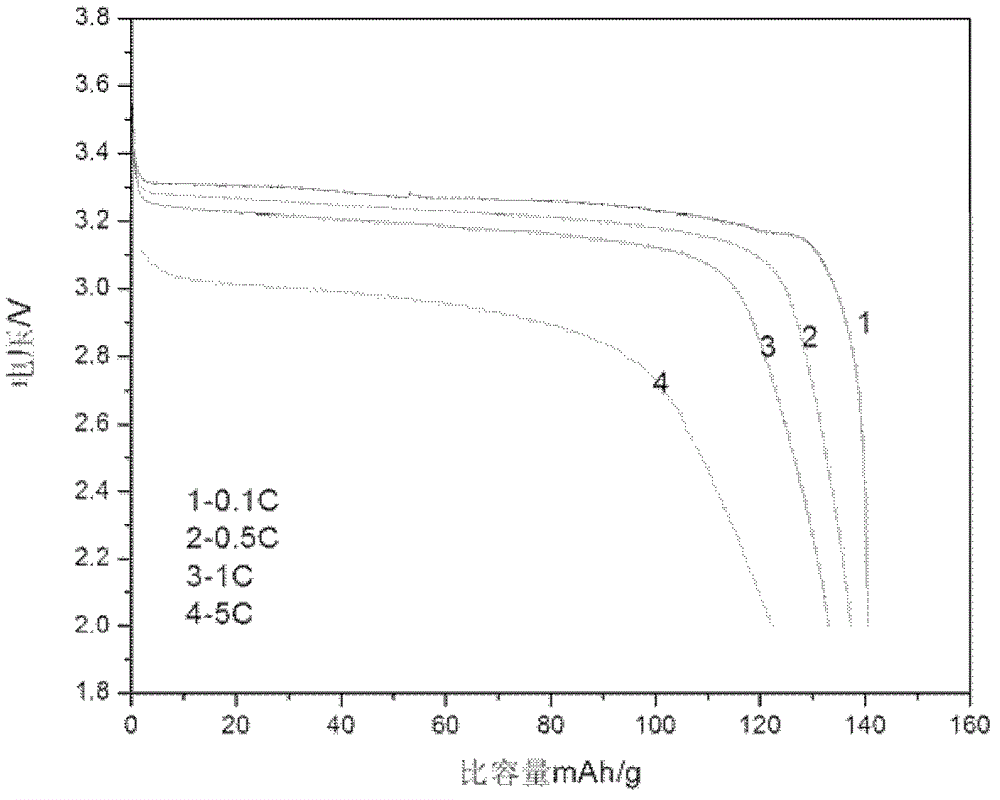
Embodiment 3
[0083] 1. The composition of electroless nickel plating solution:
[0084] Nickel sulfate 25g / L;
[0085] Sodium hypophosphite 23g / L;
[0086] Sodium acetate 30g / L;
[0087] Lactic acid 24g / L;
[0088] Glycine 5g / L;
[0089] Ammonia to adjust the pH to 4.5.
[0090] Second, the preparation of electroless nickel plating solution:
[0091] 1) Prepare lactic acid solution and sodium acetate solution respectively, and mix the two solutions to obtain mixed solution A;
[0092] 2) preparing a nickel sulfate solution, adding the mixed solution A to the nickel sulfate solution to obtain a mixed solution B;
[0093] 3) Prepare sodium hypophosphite solution and glycine solution respectively, and mix the two solutions to obtain mixed solution C;
[0094] 4) Add mixed solution C to mixed solution B, add distilled water to make up to 1 L to obtain mixed solution D, adjust the pH of mixed solution D to 4.5 with ammonia water, and obtain an electroless nickel plating solution.
[009...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Internal resistance | aaaaa | aaaaa |
| Internal resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com