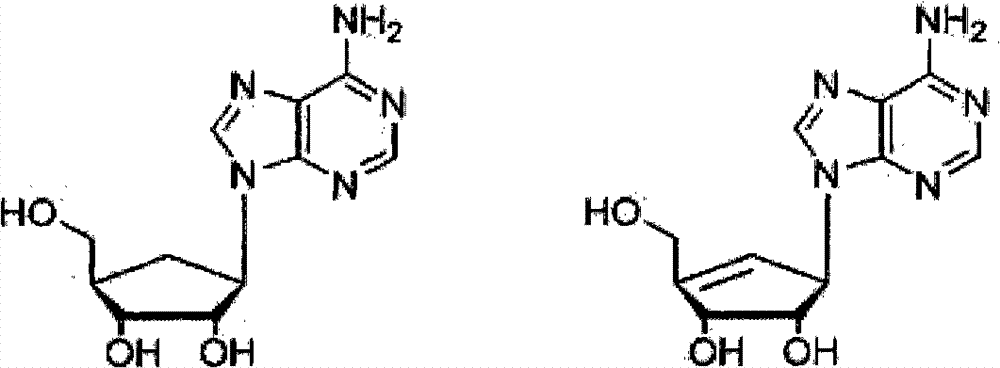
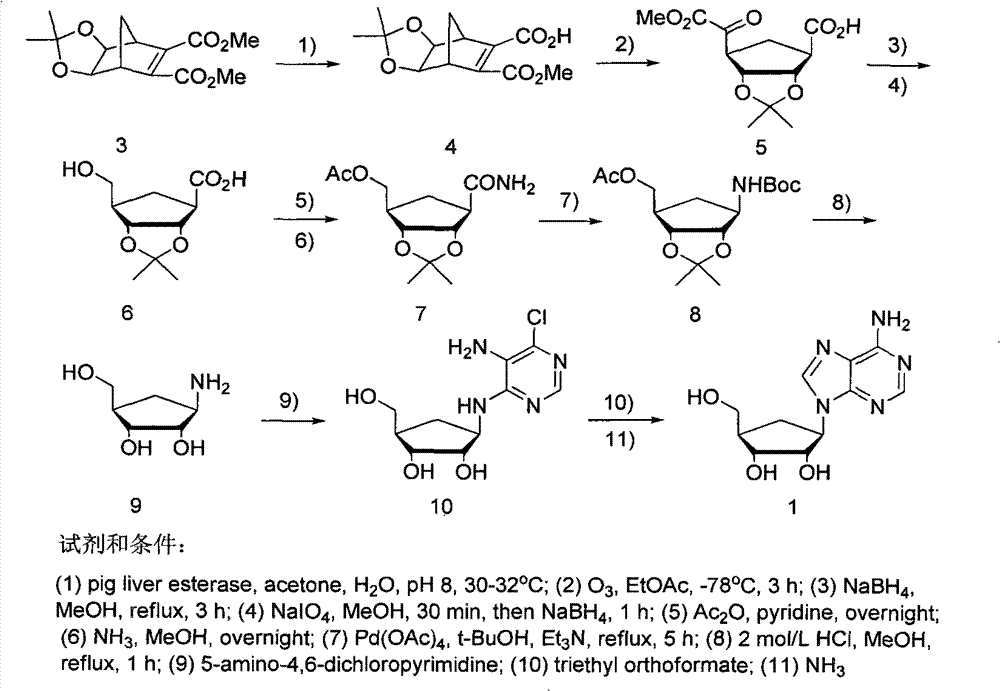
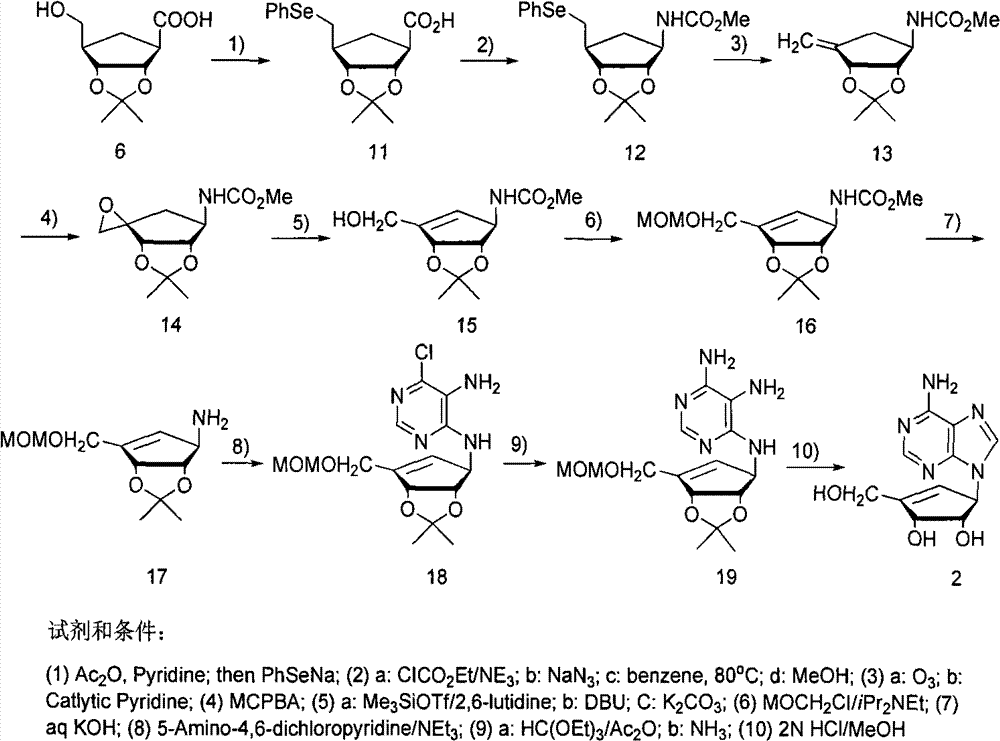
Method for synthesizing five-membered carbocyclic nucleoside
A carbocyclic nucleoside and compound technology, applied in the field of drug synthesis, can solve the problems of harsh reaction conditions, harshness, low selectivity, etc., and achieve the effect of reducing the number of uses and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Synthesis of five-membered carbocyclic uridine (B is uracil)
[0070] Compounds 21, 22, 23, 24, 25 and 26 were synthesized according to known methods (Tetrahedron: Asymmetry 2005, 16, 425), as shown in Reaction Scheme 4.
[0071] 1. Synthesis of (4R, 5S)-5-(2-(benzyloxy)acetyl)-2,2-dimethyl-1,3-dioxolane-4-carbaldehyde (compound 34)
[0072] 1) 1.13g 2-(benzyloxy)-1-((4R,5S)-5-(hydroxymethyl)-2,2-dimethyl-1,3-dioxolane-4- Base) ethanol (compound 26) (4.0mmol), 0.313g TEMPO (2.0mmol) and 5ml water are mixed, form A solution;
[0073] 2) 3.6 g of 2.2 mol / L sodium hypochlorite solution (8.0 mmol) was adjusted to pH 9.5 with acetic acid to form solution B;
[0074] 3) Under the condition of ice bath, drop solutions A and B into a 25ml three-necked flask at the same time, and the dropping speed is suitable to maintain the temperature of the reaction system at 5-10°C. After the dropping is completed, continue to stir for about 20 minutes;
[0075] 4) After the r...
Embodiment 2
[0112] Example 2: Synthesis of five-membered carbocyclic thymidine (B is thymine)
[0113] Compounds 21, 22, 23, 24, 25 and 26 were synthesized according to known methods (Tetrahedron: Asymmetry 2005, 16, 425).
[0114] Compounds 34, 35, 36, 37, 38, 39 and 40 were synthesized according to Example 1.
[0115] 1. 1-((3aS, 4R, 6aR)-6-(benzyloxymethyl)-2,2-dimethyltetrahydro-3aH-cyclopentadien[d][1,3] Synthesis of Dioxol-4-yl)-5-methylpyrimidine-2,4(1H,3H)-dione (Compound 41)
[0116] 1) Add 0.433g of compound 40 (1.0mmol) and 15ml of N,N-dimethylformamide into a 50ml three-necked flask, and stir to form a solution;
[0117] 2) Add dropwise a solution consisting of 0.63g (5.0mmol) thymine, 0.76gDBU (5.0mmol) and 10ml N,N-dimethylformamide to the above solution, then stir the reaction system at room temperature for 30min ;
[0118] 3) After concentrating the reaction system, silica gel was used for column chromatography, and the mobile phase was n-hexane-ethyl acetate (volume r...
Embodiment 3
[0122] Example 3: Synthesis of five-membered carbocyclic adenosine (B is adenine)
[0123] Compounds 21, 22, 23, 24, 25 and 26 were synthesized according to known methods (Tetrahedron: Asymmetry 2005, 16, 425).
[0124] Compounds 34, 35, 36, 37, 38, 39 and 40 were synthesized according to Example 1
[0125] 1. 9-((3aS, 4R, 6aR)-6-(benzyloxymethyl)-2,2-dimethyltetrahydro-3aH-cyclopentadien[d][1,3] Synthesis of Dioxol-4-yl)-9H-purin-6-amine (Compound 41)
[0126] Add 0.65g of compound 40 (1.5mmol) and 20ml of N,N-dimethylformamide into a 50ml three-necked flask, and stir to form a solution;
[0127]Then add dropwise a solution of 1.01g adenine (7.5mmol), 1.14gDBU (7.5mmol) and 15ml N,N-dimethylformamide to the above solution, after the dropwise addition, the reaction system was stirred at room temperature for 30min ;
[0128] After the reaction system was concentrated, column chromatography was performed on silica gel, and the mobile phase was n-hexane-ethyl acetate (70:30 b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com