High-water-solubility nuclear magnetic resonance imaging contrast agent and preparation method thereof
A nuclear magnetic resonance imaging, water-soluble technology, applied in pharmaceutical formulations, preparations for in vivo tests, organic chemistry, etc., can solve problems such as unsatisfactory water-solubility and restricted application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Preparation of tri-tert-butyl 1,4,7,10-tetraazacyclododecane-1,4,7-triacetate hydrobromide. In a 250 ml schlenk bottle, 1,4,7,10-tetraazacyclododecane (4.2 g, cyclen, laboratory-made) and anhydrous sodium acetate (6.6 g) were dissolved in N,N-dimethyl acetamide (50 ml). Then, a solution of tert-butyl bromoacetate (13 ml) in N,N-dimethylacetamide (20 ml) was added at -20°C. After the addition, react at room temperature for 1-2 days to obtain the crude product. Pour it into deionized water (500 ml), add an appropriate amount of sodium bicarbonate, and adjust the pH of the solution to 8-9. During this period, a large amount of white solids were precipitated, and the filtrate was removed by filtration. Dissolve the white solid in dichloromethane, dry it with anhydrous magnesium sulfate, then concentrate to 20 ml, add 200 ml of anhydrous ether, a white solid precipitates out, filter and collect the product.
[0028] (2) Dissolve trimethylamine hydrochloride (1.9 g) i...
Embodiment 2
[0035] (2) Dissolve dimethylhexadecyl tertiary amine hydrochloride (3.1 g) in 20 ml deionized water, add epichlorohydrin under mechanical stirring, react at 25°C-55°C for 6 h, and it becomes viscous A solid was formed, filtered, drained, and recrystallized using an ethanol\ether system to obtain the pure product 3-chloro-2-hydroxypropylhexadecyldimethylammonium chloride.
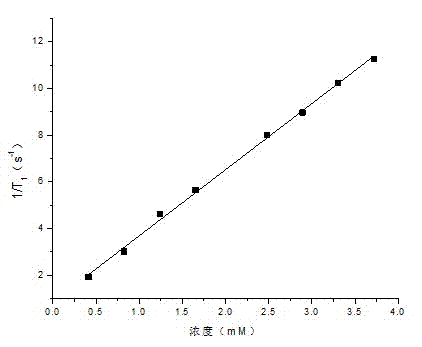
[0036] (3) Replace 3-chloro-2-hydroxypropyltrimethylammonium chloride with 3-chloro-2-hydroxypropyl hexadecyldimethylammonium chloride; the rest are the same as in Example 1. The test results show that the longitudinal relaxation time has a good linear relationship with the concentration of gadolinium ions (such as figure 2 ), and finally calculate the longitudinal relaxation rate r 1 =3.7 mM -1 the s -1 .
Embodiment 3
[0038] (2) Dissolve dimethylbehenyl tertiary amine hydrochloride (7.8 g) in 80 ml deionized water, add epichlorohydrin under mechanical stirring, and react at 35 °C-55 °C for 24 h to obtain crude The product, after dehydration and drying, was recrystallized with ethanol\ether system to obtain the pure product 3-chloro-2-hydroxypropyl behenyldimethylammonium chloride.
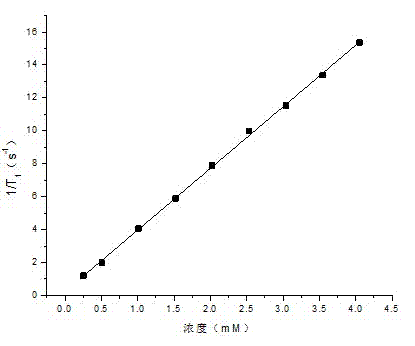
[0039] (3) Replace 3-chloro-2-hydroxypropyltrimethylammonium chloride with 3-chloro-2-hydroxypropylbehenyldimethylammonium chloride; the rest are the same as in Example 1. The test results show that the longitudinal relaxation time has a good linear relationship with the concentration of gadolinium ions (such as image 3 ), and finally calculate the longitudinal relaxation rate r 1 =8.7 mM -1 the s -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Longitudinal relaxation rate | aaaaa | aaaaa |
| Longitudinal relaxation rate | aaaaa | aaaaa |
| Longitudinal relaxation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com