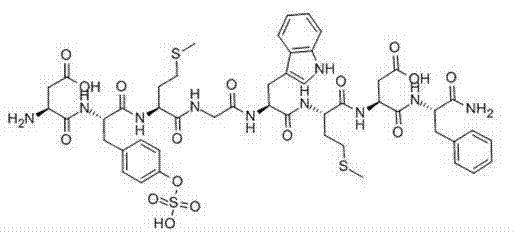
Method for synthesizing cholecystokinin octapeptide by combining solid phase method and liquid phase method
A technology of cholecystokinin and solid-phase method, which is applied in the preparation of trifluoroacetate), cholecystokinin octapeptide (sincalide including acetate), which can solve the troublesome separation of intermediate products and easily produce by-products , long preparation cycle and other issues, to achieve the effect of easy post-processing, considerable economic and practical value, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Synthesis by liquid phase method: H-Asp(otbu)-Phe-NH 2
[0039] Take 265g of Boc-Phe-OH and pour it into a round bottom flask, then add 300ml of THF (tetrahydrofuran) to dissolve it, add 133ml of NMM, at low temperature, slowly add 114ml of ClCOOET dropwise, and keep stirring. Filter out the precipitate, then collect the filtrate, add 77ml of ammonia water dropwise to the filtrate, react at room temperature for 40min, add 250ml of water, evaporate THF to dryness, then add 250ml of ethyl acetate to extract the target product, extract 3 times, then spin the filtrate to dryness, Add 300ml TFA to remove Boc, room temperature for 1 hour, add 1500ml glacial ether to precipitate, filter, dry in a positive air dryer to obtain a solid: H-Phe-NH 2 , 152g, H-Phe-NH 2 Pour into a round bottom flask, add 379g Fmoc-Asp(otbu)-OH, then dissolve with 200ml DMF, add 17.3ml DIC, 15g HOBT. Stir and react overnight at room temperature, add 250ml ethyl acetate to extract after c...
Embodiment 2
[0040] Example 2 Preparation of Fmoc-Met-CTC Resin
[0041] Dissolve 25g of Fmoc-Met-OH with 200ml of DCM and add it to a 2000ml solid-phase reactor, then add 50g of CTC Resin, then add 60ml of DIEA dropwise, blow the reaction with nitrogen for 2 hours, and then add 500ml of methanol Block for 30 minutes, filter the amino acid resin with a Buchner funnel, then wash and shrink three times with DMF, DCM, and methanol;
[0042] CTC Resin with a substitution degree of 0.3mmol / g was used as the original resin for reaction. The obtained Fmoc-Met-CTC Resin detects that the substitution degree is 0.2mmol / g. In this way, the production process with the same number of moles consumes resin, and the equivalent amount of solvent is large, which is uneconomical and not conducive to environmental protection. It is not suitable for use; CTC Resin with a substitution degree of 1.5mmol is used Carry out the reaction to obtain Fmoc-Met-CTC Resin. The detection degree of substitution is 0....
Embodiment 3
[0043] Example 3 Preparation of Fmoc-Met-CTC Resin
[0044] Choose CTC Resin with a substitution degree of 1.0mmol / g, the reaction needs to add organic base N,N-diisopropylethylamine, and the rest are the same as in Example 2, and finally the substitution degree of Fmoc-Met-CTC Resin is: 0.75 mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com