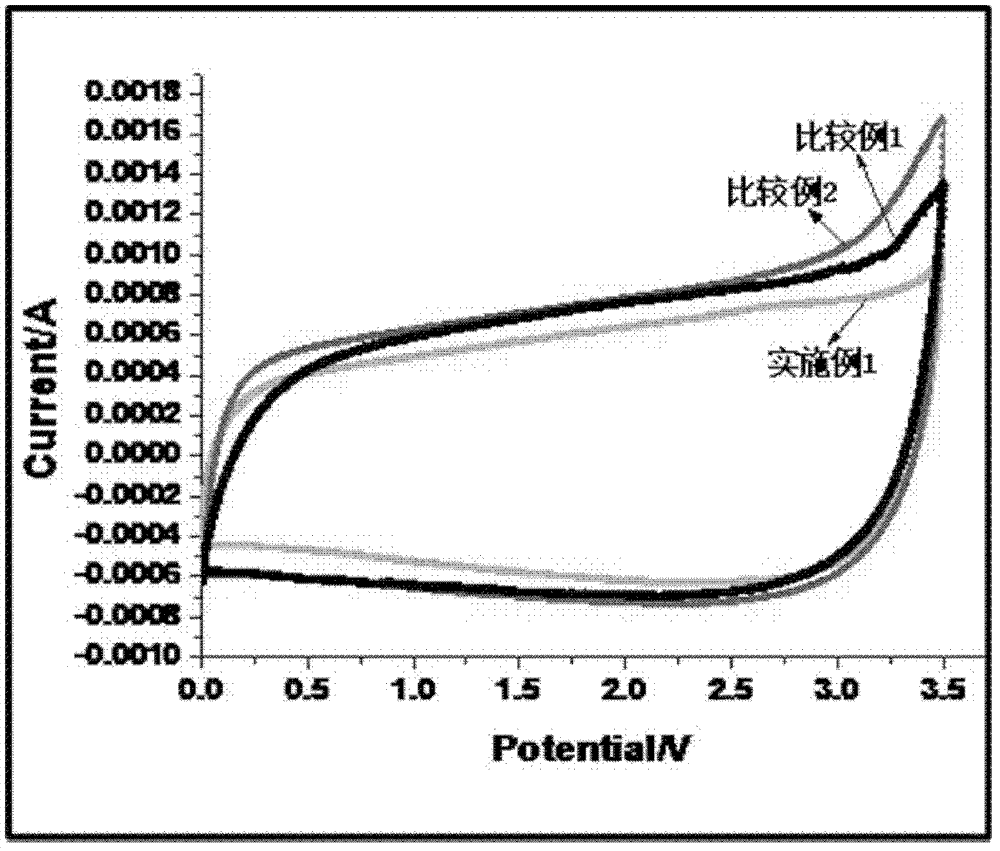
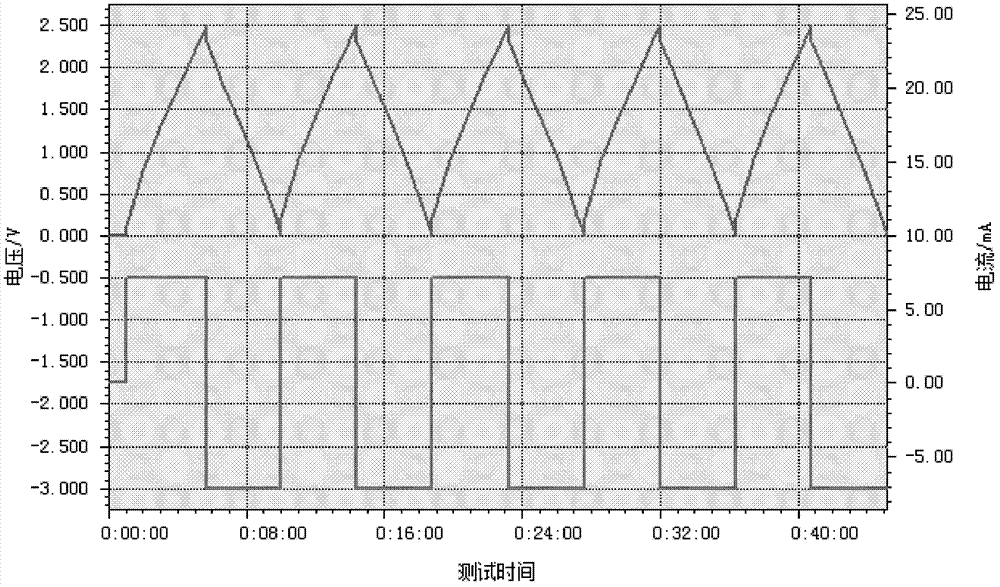
Preparation process, electrolyte and electrochemical element of electrolytic salt
An electrolyte salt and electrolyte technology, applied in electrical components, electrolytic capacitors, circuits, etc., can solve the problems of high working voltage, large internal resistance, and low ionic conductivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] (1) Synthesis of polycyclic tertiary amines
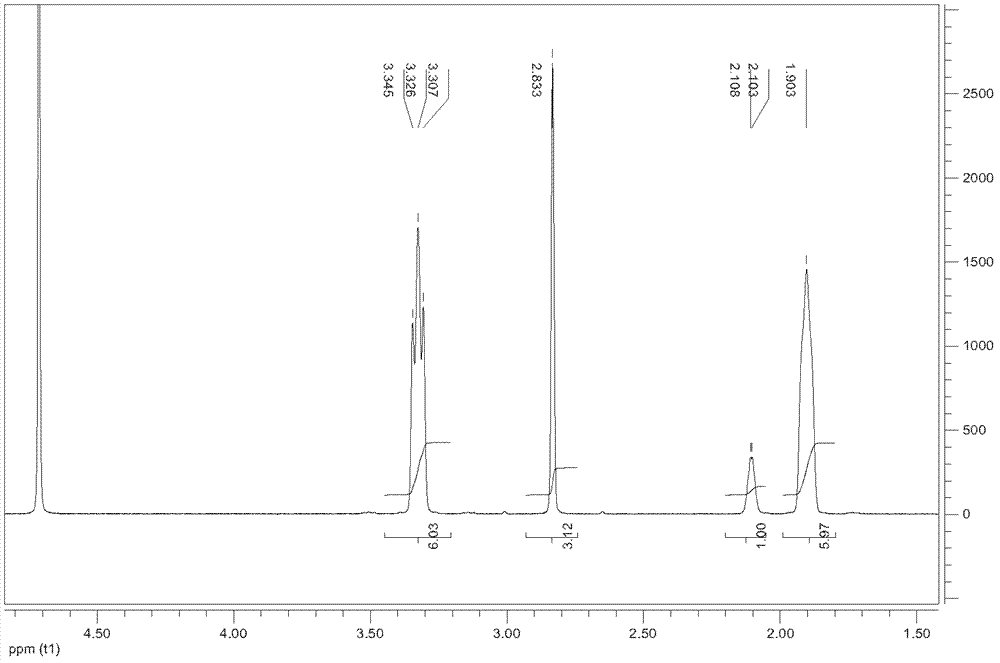
[0103] 20-40 mesh catalyst (γ-Al 2 o 3 ) 4mL, each of its two sides is filled with 1mL 20-40 mesh quartz sand, the catalyst layer and the quartz sand layer are kept at 425°C, and the alcohol compound (a=2, b=c= 1), passing through the catalyst at a speed of 2.5g / h, the obtained reaction mixture gas is dissolved in water, and separated and purified by extraction, concentration and distillation to obtain the corresponding polycyclic tertiary amine 1-azacyclo[2,2 , 2] octane, with 1 H-NMR confirmed its structure as: 1 H-NMR (CDCl 3 ): δ2.85(t, 6H), δ1.72-1.75(m, 1H), δ1.51-1.55(m, 6H).
[0104] (2) Synthesis of electrolyte salt
[0105] Mix 1-azacyclo[2,2,2]octane with dimethyl carbonate in a molar ratio (1:1.5), use methanol as a solvent, add it to a high-pressure reactor, and seal the reaction at a reaction temperature of 130°C. The reaction time was 15 hours, and the quaternary ammonium salt was obtained. The quaternar...
Embodiment 2
[0120] (1) Synthesis of polycyclic tertiary amines
[0121] 20-40 mesh catalyst (H + type molecular sieve) 4mL, each side of which is filled with 1mL 20-40 mesh quartz sand, the catalyst layer and the quartz sand layer are kept at 400°C, and the alcohol compound (a=b=c= 1), passing through the catalyst at a speed of 2.5g / h, the obtained reaction mixture gas is dissolved in water, and separated and purified by extraction, concentration and distillation to obtain the corresponding polycyclic tertiary amine 1-azacyclo[2,2 , 1] heptane, with 1 H-NMR confirmed its structure as: 1 H-NMR (D 2 O): δ2.51-2.53(ddd, 2H), δ2.34(t, 1H), δ2.41-2.443(m, 2H), δ2.19-2.20(s, 2H), δ1.38(m , 2H), δ0.94-0.95 (m, 2H).
[0122] (2) Synthesis of electrolyte salt
[0123] Dissolve 1-azacyclo[2,2,1]heptane in acetone and dissolve evenly, then slowly add iodomethane (1-azacyclo[2,2,1]heptane and iodide Methane molar ratio is 1:1), then stirred at 30°C for 3 hours. The precipitated white crystals...
Embodiment 3
[0130] (1) Synthesis of polycyclic tertiary amines
[0131] In a stainless steel tube with an inner diameter of 12mm, fill 20-40 mesh catalyst (Na + Molecular sieve) 4mL, each of its two sides is filled with 1mL 20-40 mesh quartz sand, the catalyst layer and the quartz sand layer are kept at 425°C, heated at 100°C-120°C to evaporate the alcohol compound of cyclic amine under reduced pressure (a=0, b=c =2), pass through the catalyzer at a speed of 2.5g / h, and the reaction mixture obtained is dissolved in water, and separated and purified by extraction, concentration and distillation to obtain the corresponding polycyclic tertiary amine 1-azacycle [3, 3,0]octane, with 1 H-NMR confirmed its structure as: 1 H-NMR (CDCl 3 ): δ2.95 (m, 4H), δ1.82-1.84 (m, 1H), δ1.65-1.68 (m, 4H), δ1.45-1.48 (m, 4H).
[0132] (2) Synthesis of electrolyte salt
[0133] Dissolve 1-azacyclo[3,3,0]octane in acetone, and after dissolving evenly, slowly add dimethyl sulfate (1-azacyclo[3,3,0]octane) d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com