Kallidinogenase enteric coated tablet and preparation method thereof
A technology of pancreatic kininogenase and enteric-coated tablets, which is applied in the fields of pill delivery, pharmaceutical formulation, peptide/protein composition, etc., and can solve the problem of non-compliance of content uniformity of finished products, non-compliance of content uniformity and unstable drug quality and other issues to achieve the effect of avoiding titer loss, less potency unit loss, and improving content and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
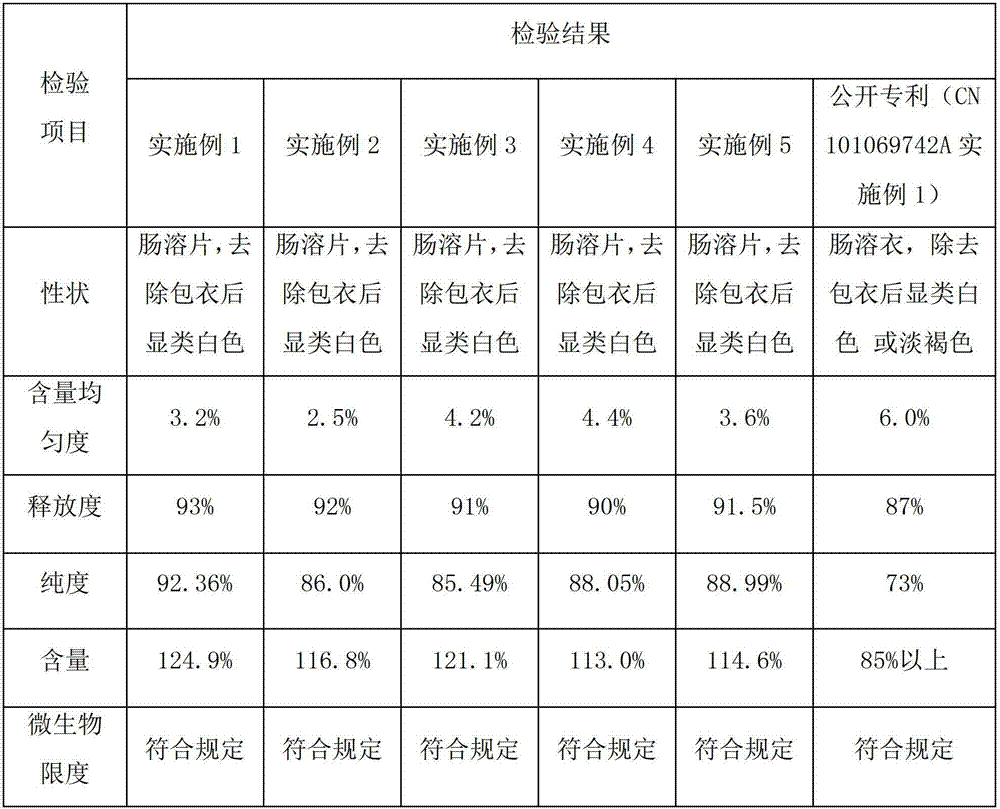
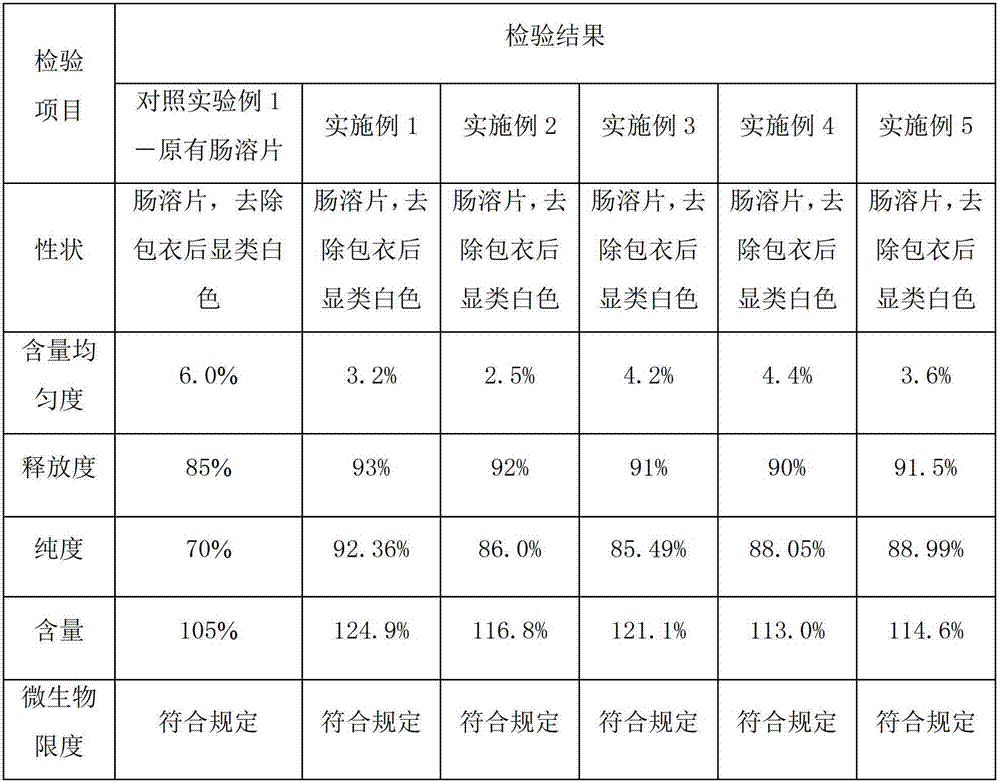
Embodiment 1
[0031] Example 1 Preparation of pancreatic kininogenase tablets
[0032] A pancreatic kininogenase enteric-coated tablet, every 1000 tablets includes the following components:
[0033] Pancreatic kininogenase: 140,000 units
[0034] Starch: 130g
[0035] Microcrystalline Cellulose: 20g
[0037] Hypromellose: 8g
[0039] Enteric Film Coating Premix (Ahua) 24g
[0040] Preparation:
[0041] 1. Preparation: Grind excipients such as pancreatic kininogenase, starch, microcrystalline cellulose, sodium carbonate, hypromellose, and magnesium stearate through an 80-mesh sieve.
[0042] 2. Prepare blank granules: Mix the weighed auxiliary materials according to the prescription amount, add purified water and stir evenly to make soft materials, pass through a 20-mesh sieve to prepare blank granules. Place the granules in a hot air circulation oven, and dry them with hot air below 50°C. The dried blank granules are size...
Embodiment 2
[0048] Example 2 Preparation of pancreatic kininogenase tablets
[0049] A pancreatic kininogenase enteric-coated tablet, every 1000 tablets includes the following components:
[0050] Pancreatic kininogenase: 90,000 units
[0051] Lactose: 140g
[0052] Microcrystalline Cellulose: 15g
[0053] Sodium Alginate 25g
[0054] Hypromellose: 6g
[0056] Enteric film coating premix (Yingmao) 28
[0057] 1. Preparation: Grind excipients such as pancreatic kininogenase, lactose, microcrystalline cellulose, sodium alginate, hypromellose, and magnesium stearate through an 80-mesh sieve.
[0058] 2. Prepare blank granules: Mix the weighed auxiliary materials according to the prescription amount, add purified water and stir evenly to make soft materials, pass through a 20-mesh sieve to prepare blank granules. Place the granules in a hot air circulation oven, and dry them with hot air below 50°C. The dried blank granules are sized with a 20-mesh stainles...
Embodiment 3
[0063] Example 3 Preparation of pancreatic kininogenase tablets
[0064] A pancreatic kininogenase enteric-coated tablet, every 1000 tablets includes the following components:
[0065] Pancreatic kininogenase: 120,000 units
[0066] Lactose: 120g
[0067] Sodium starch glycolate: 18g
[0069] Hypromellose: 7g
[0071] Enteric Film Coating Premix (Ahua) 20g
[0072] 1. Preparation: Grind excipients such as pancreatic kininogenase, lactose, sodium starch glycolate, calcium chloride, hypromellose, and magnesium stearate through an 80-mesh sieve.
[0073] 2. Prepare blank granules: Mix the weighed auxiliary materials according to the prescription amount, add purified water and stir evenly to make soft materials, pass through a 20-mesh sieve to prepare blank granules. Place the granules in a hot air circulation oven, and dry them with hot air below 50°C. The dried blank granules are sized with a 20-mesh stainless s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com