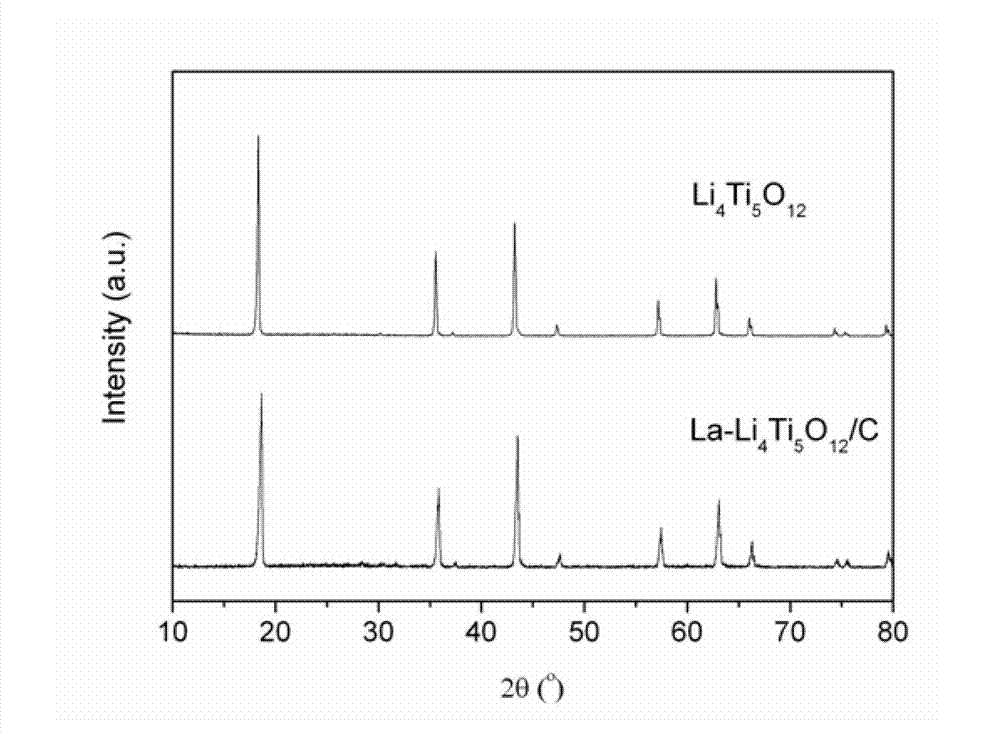
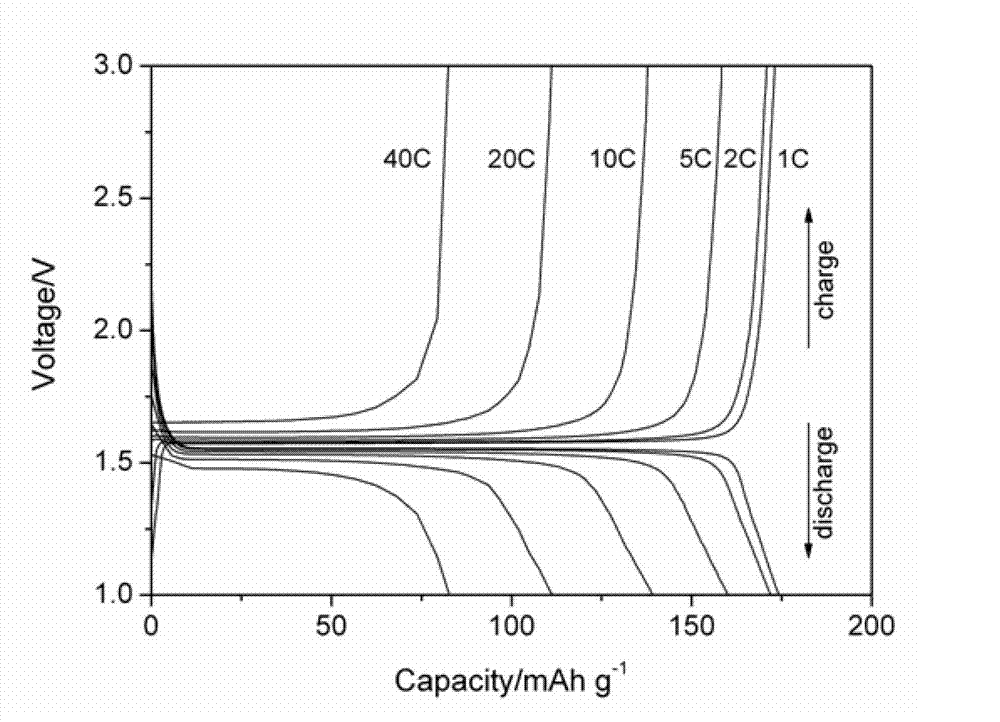
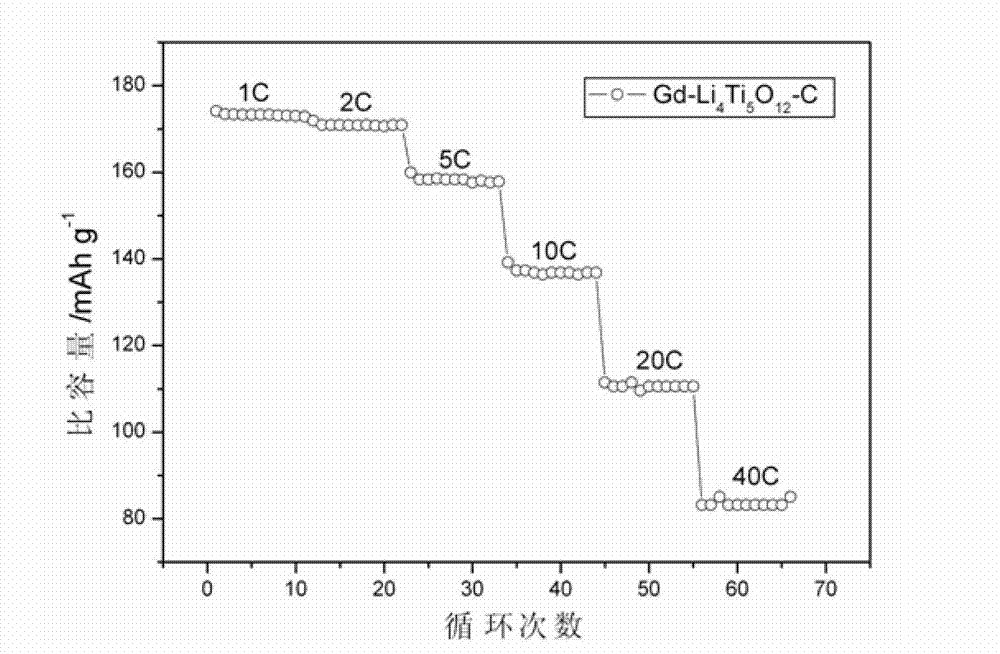
Electrostatic spinning method for preparing rare-earth metal doped nanometer lithium titanate
A technology of nano-lithium titanate and electrospinning, which is applied in the direction of circuits, electrical components, battery electrodes, etc., can solve the problems of particle agglomeration, inability to form a gel three-dimensional network structure, poor electrochemical performance, etc., and achieve improved particle size. Effects of agglomeration, improvement of electrochemical performance, and improvement of electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Put 0.6g of polyvinylpyrrolidone (PVP K90) into 9mL of absolute ethanol and stir until completely dissolved. According to the molar ratio of Li:Ti=4.2:5, 1.702g of tetra-n-butyl titanate and 0.277g of lithium acetate were put into the obtained polymer solution, and 1.778g of citric acid was added at the same time, and stirred until the solution was clear. Obtain solution A; according to the molar ratio of La:Ti=0.02:5, add 0.0033g of lanthanum oxide into 3mL of nitric acid and completely dissolve to obtain solution B. Mix solution A and solution B, stir at room temperature for 2 hours, add 0.1 mL of 1-methyl-2-pyrrolidone and continue stirring at room temperature for 1.5 hours to obtain an electrospinning solution. The parameters of the electrospinning process were selected as follows: the flow rate was 0.3mL / hr, the working voltage was 15KV, and the distance between the receiving plates was 15cm. Electrospinning was carried out. After spinning a 2mm white film, it was ...
Embodiment 2
[0029] Put 2.48g of polyoxyethylene-polyoxypropylene-polyoxyethylene triblock copolymer (Pluronic P123) into 17.73mL of absolute ethanol, and stir until completely dissolved. Then according to the molar ratio of Li:Ti=1:1, 1.702g of tetra-n-butyl titanate and 0.345g of lithium nitrate were put into the prepared polymer solution, and 2.260g of triethanolamine was added at the same time, and stirred until the solution was clear. Obtain solution A; according to the molar ratio of Nd:Ti=0.1:5, add 0.0168g of neodymium oxide into 7.09mL of acetic acid to completely dissolve and obtain solution B. Mix solution A and solution B, stir at room temperature for 3 hours, add 0.50 mL of dichloromethane and continue stirring at room temperature for 1 hour to obtain an electrospinning solution. The electrospinning process parameters were selected as follows: the flow rate was 1.0mL / hr, the working voltage was 15KV, and the receiving plate distance was 30cm. Electrospinning was performed. Aft...
Embodiment 3
[0031] Put 4.04g of polyvinyl butyral into 13.46mL of absolute ethanol and stir until completely dissolved. According to the molar ratio of Li:Ti=4.8:5, Gd:Ti=0.001:5, 1.421g of tetraisopropyl titanate, 0.178g of lithium carbonate, and 0.0004g of gadolinium nitrate were put into the prepared polymer solution, Add 6.73 mL of hydrochloric acid and stir until the solution is clear. Finally, 0.30 mL of acetone was added and stirring was continued at room temperature for 1 hour to obtain an electrospinning solution. The parameters of the electrospinning process were selected as follows: the flow rate was 0.2mL / hr, the working voltage was 10KV, and the distance between the receiving plates was 10cm. Electrospinning was performed. After spinning a 1mm white film, it was vacuum-dried and stabilized at room temperature for 6 hours. The film was removed and calcined at a high temperature of 700° C. for 15 hours in a tube furnace under a nitrogen atmosphere to obtain a gadolinium-doped ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com