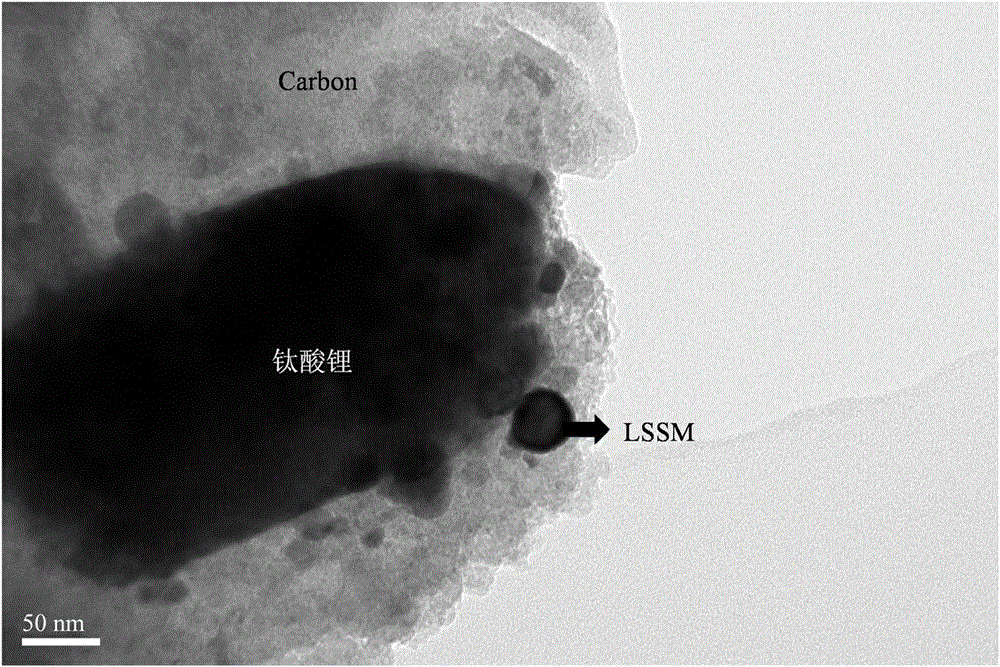
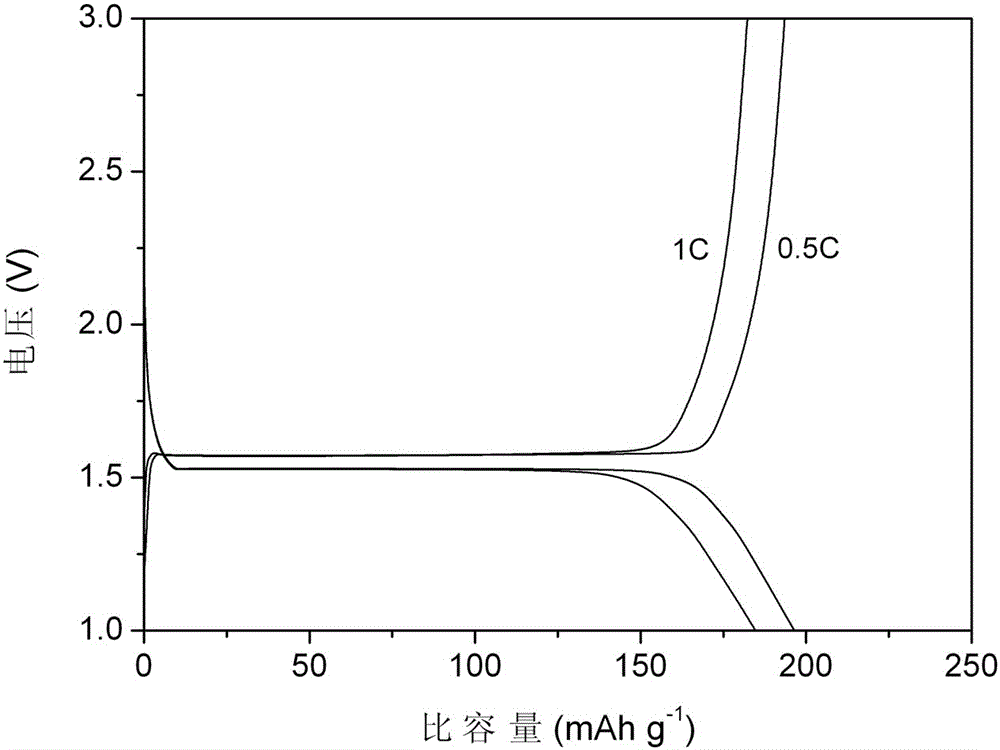
A preparation method of nano-lithium titanate coated with double high-conductivity materials
A technology with high conductivity and material packaging, applied in nanotechnology, circuits, electrical components, etc., can solve the problems of electrolyte consumption, low initial Coulombic efficiency, low initial charge and discharge efficiency, and limitations, and achieve superior fast charge and discharge performance, Effect of improving particle agglomeration and good cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] First, premix 300 ml of ethanol and 30 ml of water according to the volume ratio of 1:0.1 to form a mixed solvent, and then add 15 ml of HNO 3As an inhibitor of the follow-up reaction; the soluble compounds of Li and Ti are compounded according to the molar ratio of Li:Ti=4.2:5, and 25.52g of tetra-n-butyl titanate (analytically pure) is weighed, and 2.33g of carbonic acid Lithium (analytical pure), added to the previous alcohol-water-acid mixture, stirred by a magnetic heating stirrer until completely dissolved; then 20g of ethylenediaminetetraacetic acid and 30g of citric acid were added to the pre-mixed metal ion solution After mixing evenly, add 100 ml of ammonia water dropwise to adjust the pH value to 8, and continue to stir; after the above mixed solution is stirred evenly to form a sol, heat and stir at 80°C until it reaches a gel state; :Sr:Sc:Mn=0.5:0.5:0.1:0.9 molar ratio for batching, weigh 9.48 g lanthanum acetate (analytical pure), 9.69 g strontium nitrate...
Embodiment 2
[0030] According to the volume ratio of 1:0.1, 300 ml of ethanol and 30 ml of water are premixed to form a mixed solvent, and then 15 ml of HCl is added as an inhibitor of the subsequent reaction; the soluble compound of Li and Ti is compounded according to Li:Ti=4.2:5 The molar ratio is carried out batching, and the tetraisopropyl titanate (analytical pure) of 21.31g, the lithium acetate (analytical pure) of 6.43 g are joined in the previous alcohol-water-acid mixture, stirred by a magnetic heating stirrer, Until it is completely dissolved; then add 20 g of ethylenediaminetetraacetic acid and 40 g of citric acid into the premixed metal ion solution, mix well, add 100 ml of ammonia water dropwise to adjust the pH value to 9, and continue stirring; wait for the above mixing After the solution was stirred evenly to form a sol, it was then heated and stirred at 80°C until it reached a gel state; the La, Sr, Sc and Mn compounds were mixed according to the molar ratio of La:Sr:Sc:Mn...
Embodiment 3
[0032] According to the volume ratio of 1:0.2, pre-mix 300 ml of ethanol and 60 ml of water to form a mixed solvent, and then add 30 ml of HNO 3 As an inhibitor of the follow-up reaction; the soluble compounds of Li and Ti are compounded according to the molar ratio of Li:Ti=4.4:5, and 25.52 g of tetra-n-butyl titanate (analytical pure), 4.55 g of nitric acid Lithium (analytically pure), was added to the previous alcohol-water-acid mixture, stirred by a magnetic heating stirrer until it was completely dissolved; then 20 g of ethylenediaminetetraacetic acid and 60 g of citric acid were added to the pre-mixed metal In the ionic solution, after mixing evenly, add 110ml of ammonia water dropwise to adjust the pH value to 7, and continue to stir; after the above mixed solution is stirred evenly to form a sol, heat and stir at 80°C until it is in a gel state; La:Sr:Sc:Mn=0.5:0.5:0.1:0.9 molar ratio for batching, weigh 9.48 g lanthanum oxalate (analytical pure), 5.27 g strontium oxal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com