Fusion protein of human serum albumin and lipolysis structural domain of human growth hormone
A human growth hormone and fusion protein technology, applied in the biological field, can solve the problems of difficult to exert drug effect, short half-life, easy to be hydrolyzed by oral administration, etc., and achieve the effects of being beneficial to clinical use, long half-life and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Cloning of HSA cDNA
[0037] HSAcDNA without signal peptide coding sequence was obtained from human fetal liver cDNA library by PCR.
[0038] The designed primers are:
[0039] HSA up: 5'ATGC GAATTC GATGCACACAAGAGTGAGGTT 3'
[0040] HSA dn: 5'ATGC GGATCC TAAGCCTAAGGCAGCTTGACT 3’
[0041] The upstream and downstream primers introduced EcoRI and BamHI sites and protective bases respectively, and the underlined part is the endonuclease recognition sequence.
[0042] PCR reaction conditions: In 100 μL reaction system, add 1.5 μL liver tissue cDNA (obtained by reverse transcription after extraction by RNA extraction kit), 1.5 μL each of 20 μmol / L upstream and downstream primers, 10 mmol / L dNTP (deoxynucleotide ) 1 μL, 10 μL of 10× reaction buffer, 0.5 μL of Taq DNA polymerase, and ddH for the rest 2 O make up.
[0043] Using EPPENDORF’s (model Matstercycler Gradient) PCR instrument, the PCR reaction conditions were pre-denaturation at 94°C for 5 min; dena...
Embodiment 2
[0046] Embodiment 2: Construction of fusion expression vector
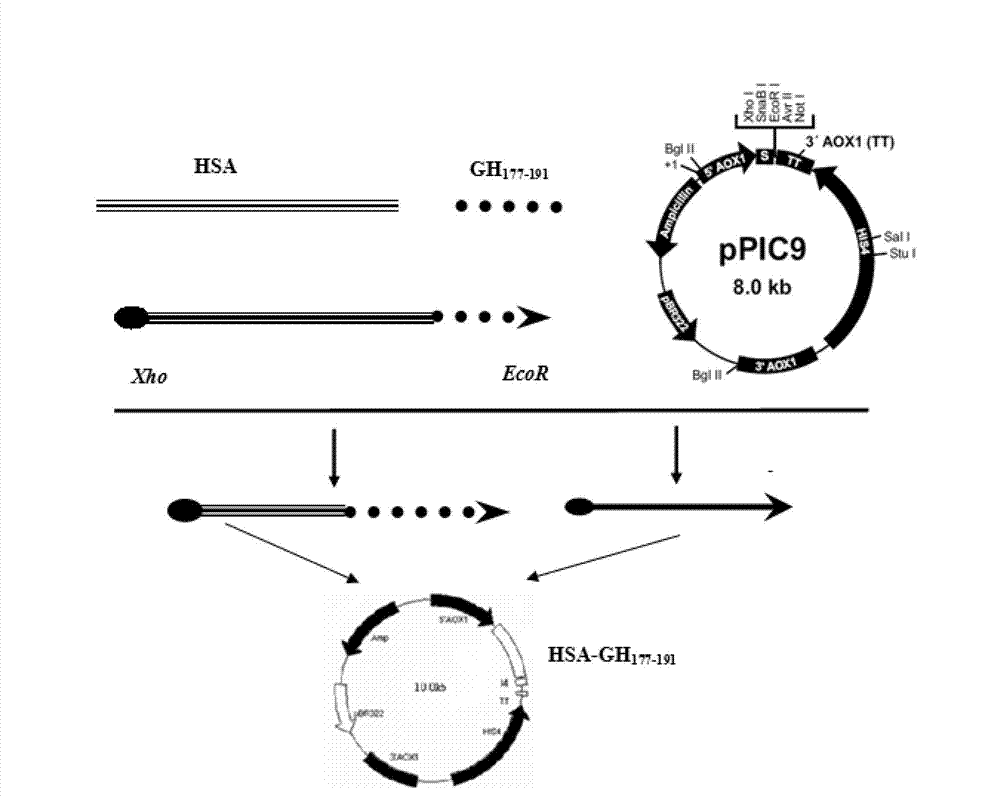
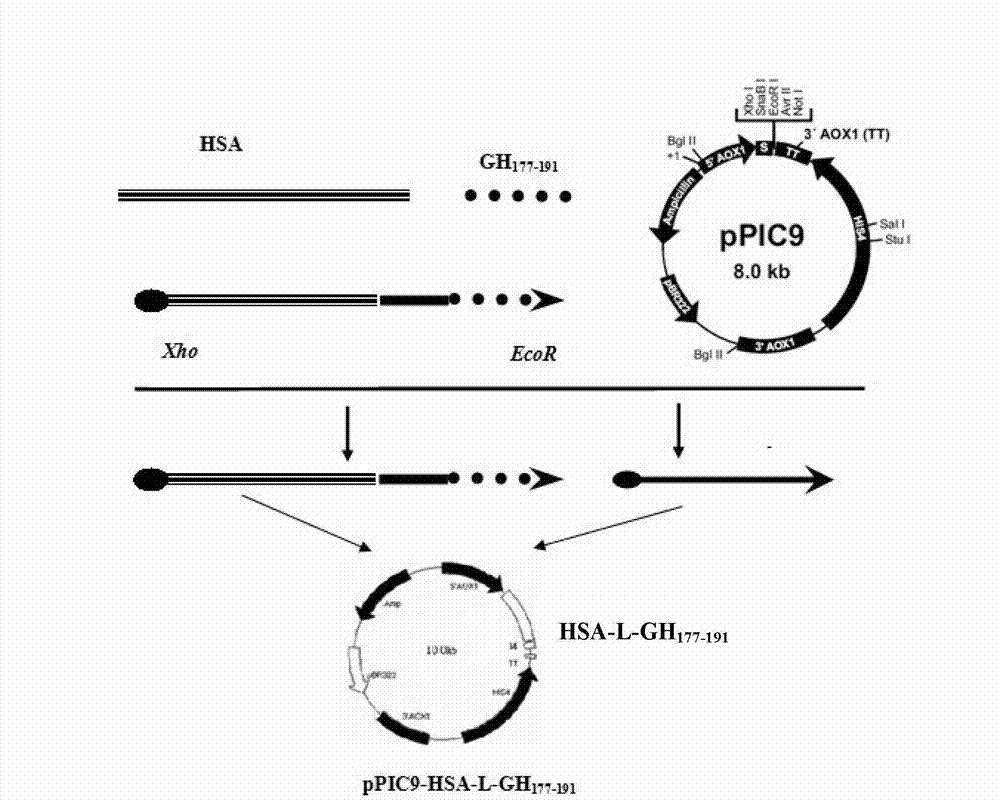
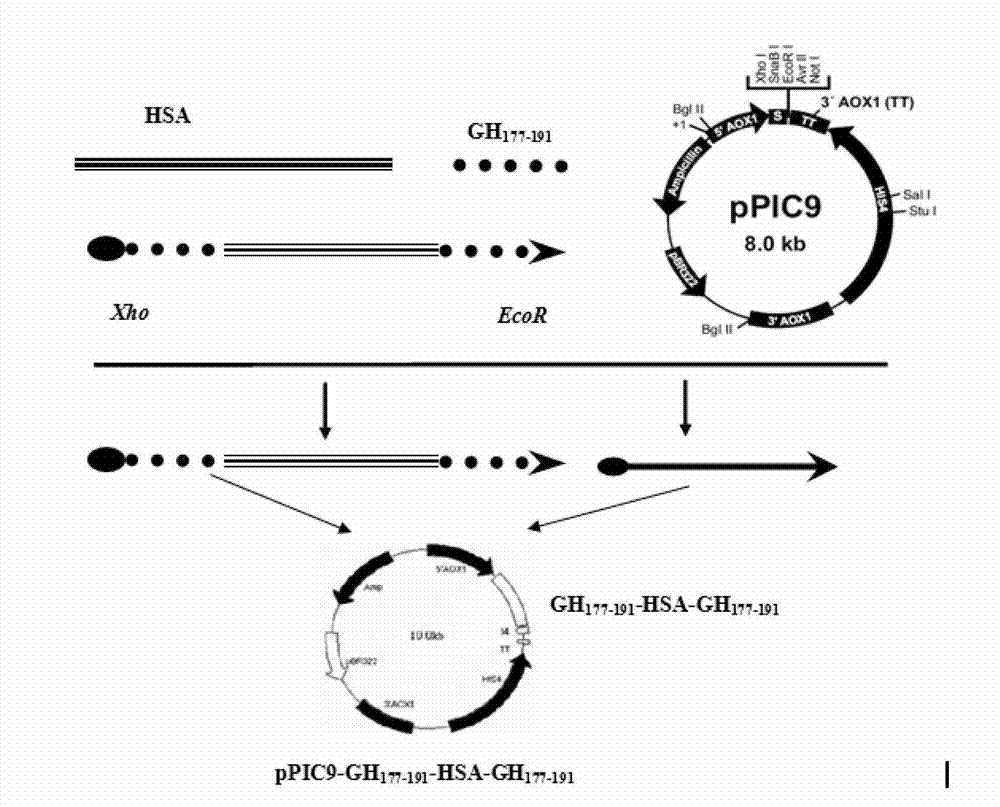
[0047] hGH 177-191 It is the 15 AAs at the C-terminus of hGH, and its amino acid sequence is: H 2 N-Leu-Arg-Ile-Val-Gln-Cys-Arg-Ser-Val-Glu-Gly-Ser-Cys-Gly-Phe-OH. Derivation of hGH from yeast-preferred codons 177-191 The nucleotide sequence is: ctg aga atc gtt cag tgc aga tct gtt gag ggt tct tgt ggt ttc, the recombinant plasmid constructed, such as figure 1 , 2 , 3 shown.
[0048] Using the pGEM-T-HSA plasmid constructed in Example 1 as a template, HSA gene and hGH were amplified in vitro by PCR 177-191 Fragment fusion gene. The fusion gene HSA-L-hGH was amplified by using primers 1 and 4 as upstream and downstream primers 177-191 , using primers 3 and 2 as upstream and downstream primers to amplify the fusion gene hGH 177-191 -HSA-hGH 177-191 . Using primers 1 and 2 as upstream and downstream primers to amplify the fusion gene HSA-hGH 177-191 .
[0049] Table 1: Primers for recombinant expression v...
Embodiment 3
[0072] Example 3 Transformation of Pichia pastoris GS115 cells, screening and expression with recombinant expression plasmids
[0073] The electroporation transformation and clone screening methods refer to the Invitrogen Pichia experimental manual, and the host cell is the Pichia GS115 strain.
[0074] Select the appropriate linearization site according to the target gene and vector, select SalI for enzyme digestion, recover the fragment containing the fusion protein gene, and use the recovered fragment to transform Pichia pastoris GS115 cells by electric shock. The transformed GS115 was spread on an RDB agar plate containing sorbitol, cultured at 30°C for 1-2 days, and His+ clones were picked. Inoculate into a 250ml Erlenmeyer flask filled with 25ml BMGY medium, culture at 30°C for 48 hours at 250 rpm to OD 600 =2~6. After centrifugation, the cells were collected to make OD with BMMY medium 600 = about 1.0 (the amount of BMMY medium is about 100-200 mL), continue to cultu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com