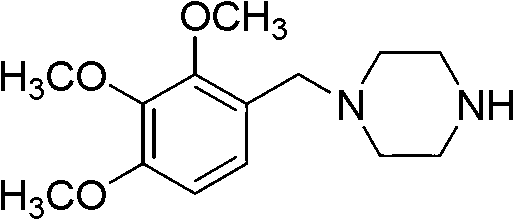
Preparation method of trimetazidine
A technology of trimetazidine and piperazine, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of difficult industrial production, unfavorable scale-up, unfavorable production, etc., and achieve the effects of high yield, enhanced safety, and reduced synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Put 180g of 2,3,4-trimethoxybenzaldehyde, 553g of piperazine, and 800mL of methanol into the reactor, quickly raise the temperature of the reaction system to 63±3°C, then add 733g of formic acid into the reaction system, raise the temperature to 85°C and keep At this temperature for 3 hours, methanol was distilled off under reduced pressure, then 800mL of 40% sodium hydroxide solution was added to the reaction system, the temperature was raised to 100°C and kept at this temperature for 1 hour, the pH was adjusted to 11, the temperature was lowered, and used at 12±2°C 8N hydrochloric acid to adjust the pH value of the material liquid to 1, wash 3 times with 200mL of dichloromethane each, and then neutralize the aqueous phase with 520g40% sodium hydroxide to a pH value of 12, extract 3 times with 100mL of toluene each, anhydrous sulfuric acid Magnesium drying, vacuum rotary evaporation to obtain trimetazidine 217.3g, yield: 89%.
Embodiment 2
[0038] Put 180g of 2,3,4-trimethoxybenzaldehyde, 620g of piperazine, and 800mL of toluene into the reactor, quickly raise the temperature of the reaction system to 103±3°C, then add 648g of formic acid into the reaction system, raise the temperature to 105°C and keep At this temperature for 3.5 hours, toluene was evaporated under reduced pressure, then 800mL of 40% sodium hydroxide solution was added to the reaction system, the temperature was raised to 105°C and kept at this temperature for 1.5 hours, the pH was adjusted to 12, the temperature was lowered, and used at 8±3°C Adjust the pH value of the feed solution to 1.5 with concentrated hydrochloric acid, wash with 200mL of chloroform three times, neutralize the water phase with 490g of 40% sodium hydroxide to pH 10, extract with 100mL of toluene three times, and dry over anhydrous magnesium sulfate , 202.7 g of trimetazidine was obtained by rotary evaporation under reduced pressure, yield: 83%.
Embodiment 3
[0040] Put 180g of 2,3,4-trimethoxybenzaldehyde, 720g of piperazine, and 800mL of toluene into the reactor, quickly raise the temperature of the reaction system to 103±3°C, then add 648g of formic acid into the reaction system, raise the temperature to 110°C and keep At this temperature for 5 hours, toluene was evaporated under reduced pressure, then 800mL of 40% sodium hydroxide solution was added to the reaction system, the temperature was raised to 105°C and maintained at this temperature for 1.5 hours, the pH was adjusted to 13, the temperature was lowered, and used at 5-10°C Concentrated hydrochloric acid to adjust the pH value of the feed solution to 2, wash with 200mL of chloroform for 3 times, then neutralize the water phase with 490g of 40% sodium hydroxide until the pH value is 10, extract with 100mL of toluene for 3 times, and dry over anhydrous magnesium sulfate , 202.7 g of trimetazidine was obtained by rotary evaporation under reduced pressure, yield: 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com