Doped conjugated polymers, devices, and methods of making devices
A technology of conjugated polymers and dopants, which is applied in the direction of electric solid-state devices, capacitor components, hybrid capacitor electrolytes, etc., can solve problems such as hole injection layer and hole transport layer, and achieve improved current density and luminescence Effect of performance, performance improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
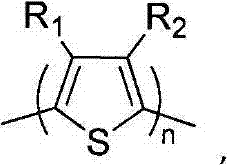
[0233] Embodiment 1: the synthetic method of 3,4-two (2-(2-butoxyethoxy) ethoxy) thiophene
[0234]
[0235] To a dry three-neck round bottom flask (1 L) purged with nitrogen was added 420 mL of butyl carbitol followed by 29 g of sodium metal (shavings) to facilitate rapid dissolution. The sodium metal was washed with hexane before adding to butyl carbitol. The reaction mixture was stirred at room temperature for about 30-45 minutes. The reaction mixture was then heated to 90°C to complete the reaction of the metal with butyl carbitol.
[0236] To the mixture was added 75 g of 3,4-dibromothiophene, followed by 4.45 g of CuBr and 0.51 g of KI. The reaction mixture was heated to 90-100 °C for 24 hours. GC-MS of the reaction mixture indicated greater than 98% conversion. The reaction mixture was diluted with about 500 mL of tert-butyl methyl ether (MTBE). The reaction mixture was then filtered on a 90cm x 1cm thick pad of silica gel. Additional filtration was performed...
Embodiment 2
[0238] Example 2: Debromination of 3,4-bis(2-(2-butoxyethoxy)ethoxy)thiophene monomer
[0239]
[0240] 20 g of 3,4-bis(2-(2-butoxyethoxy)ethoxy)thiophene monomer was dissolved in 90 mL of chloroform. Then 90 mL of glacial acetic acid was added to the mixture. The solution was cooled to about 0-5°C using an ice bath, and 19.5 g of N-bromosuccinimide was added in small portions over 2-3 minutes. The reaction mixture darkened rapidly. The reaction was continued for about 2 hours at room temperature, and then neutralized with a diluted solution of sodium bicarbonate.
[0241] The reaction mixture was transferred to a separatory funnel and further diluted with 1 L MTBE. The organic layer was washed with 1 x 300 mL deionized water followed by 2 x 300 mL 1M sodium thiosulfate solution. For the thiosulfate wash, the organic layer was stirred for 30 minutes in a beaker before separation. Finally, the organic layer was washed 2x 200 mL with brine, washed with anhydrous MgSO ...
Embodiment 3
[0242] Example 3: Polymerization of 2,5-dibromo-3,4-bis(2-(2-butoxyethoxy)ethoxy)thiophene (I)
[0243]
[0244] A 1 L three-neck round bottom flask was evacuated while heating and purging three times with nitrogen. To this flask was added 400 mL of anhydrous tetrahydrofuran (THF) from MBraun solvent delivery system. 22.59 g of 2,5-dibromo-3,4-bis(2-(2-butoxyethoxy)ethoxy)thiophene monomer was diluted with about 80 mL of anhydrous THF and added to a 1 L reaction flask. The flask was rinsed with 20 mL of anhydrous THF, and the rinse was added to the reactor. Next, 36.2 mL of 1.0 M i-PrMgCl.LiCl in THF was injected into the reaction mixture via syringe. The reaction mixture was stirred at room temperature for 45 minutes. Suspended with 0.22 g NiCl via syringe 2 .dppp in 6 mL dry THF. The reaction mixture turned opaque purple within minutes.
[0245] The reaction mixture was refluxed overnight and then added to 4 L of ethanol. The reaction mixture was concentrated by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com