Lithium manganate material and preparation method thereof
A technology of lithium manganese oxide and manganese salt, which is applied in the field of lithium manganate material and its preparation, and can solve the problems of commercialization limitations, poor cycle performance of lithium-ion batteries, poor stability and reversibility of lattice structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
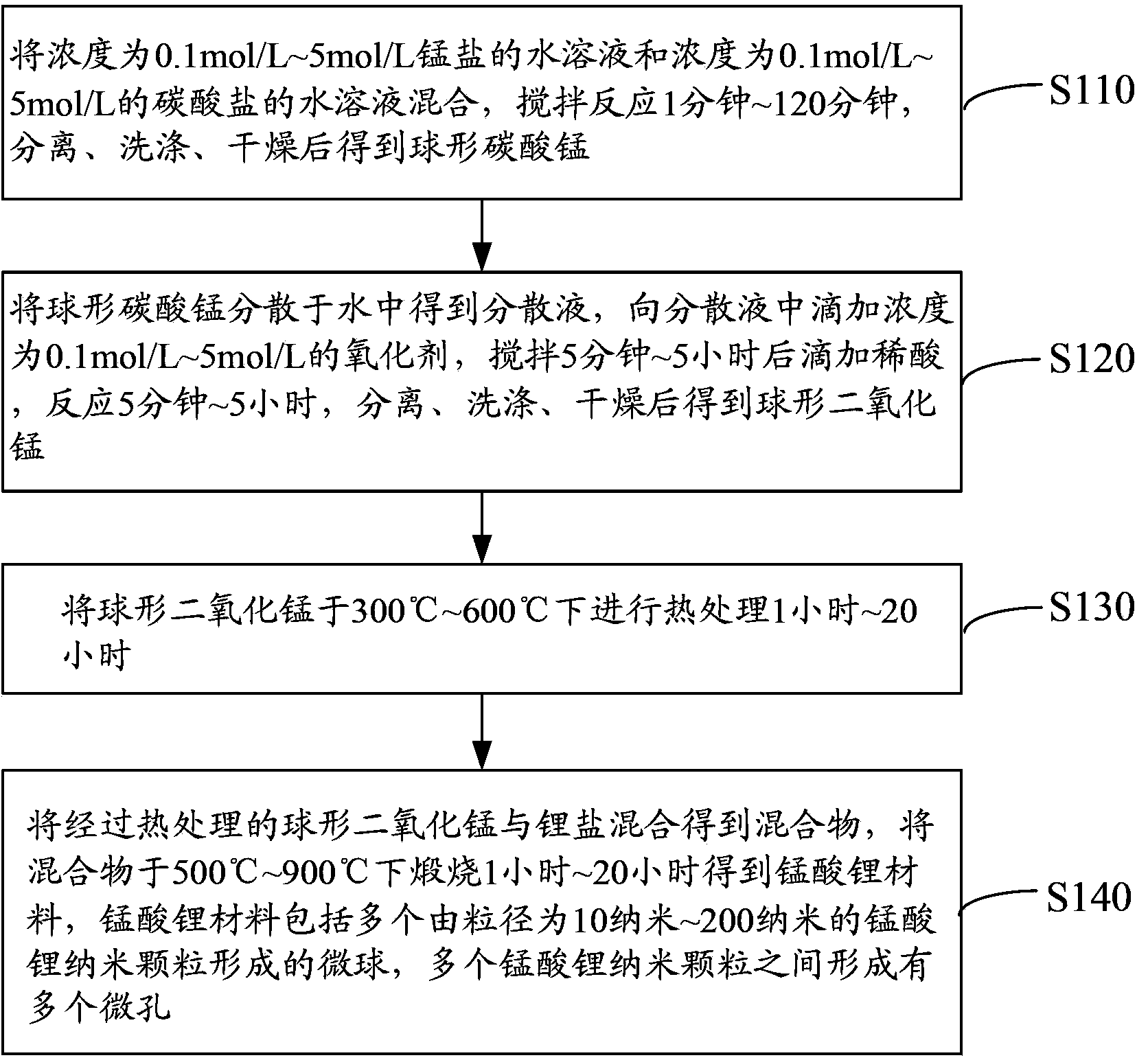
[0034] see figure 1 , the preparation method of the lithium manganate material of one embodiment, comprises the following steps:
[0035] Step S110: Mix an aqueous solution of manganese salt with a concentration of 0.1 mol / L to 5 mol / L and an aqueous solution of carbonate with a concentration of 0.1 mol / L to 5 mol / L, stir and react for 1 to 120 minutes, separate, wash, and dry After obtaining spherical manganese carbonate.
[0036] The manganese salt is manganese acetate (C 4 H 6 MnO 4 ·4H 2 O), manganese nitrate (Mn(NO) 3 ) 2 ·4H 2 O), manganese sulfate (MnSO) 4 ·H 2 O) or manganese chloride (MnCl 2 ).
[0037] Carbonate is sodium carbonate (Na 2 CO 3 ), sodium bicarbonate (NaHCO 3 ), potassium carbonate (K 2 CO 3 ), potassium bicarbonate (KHCO 3 ), ammonium carbonate ((NH 4 ) 2 CO 3 ) or ammonium bicarbonate (NH 4 HCO 3 ).
[0038] These manganese salts and carbonates are relatively cheap, and are water-soluble inorganic salts, so that the reaction can...
Embodiment 1
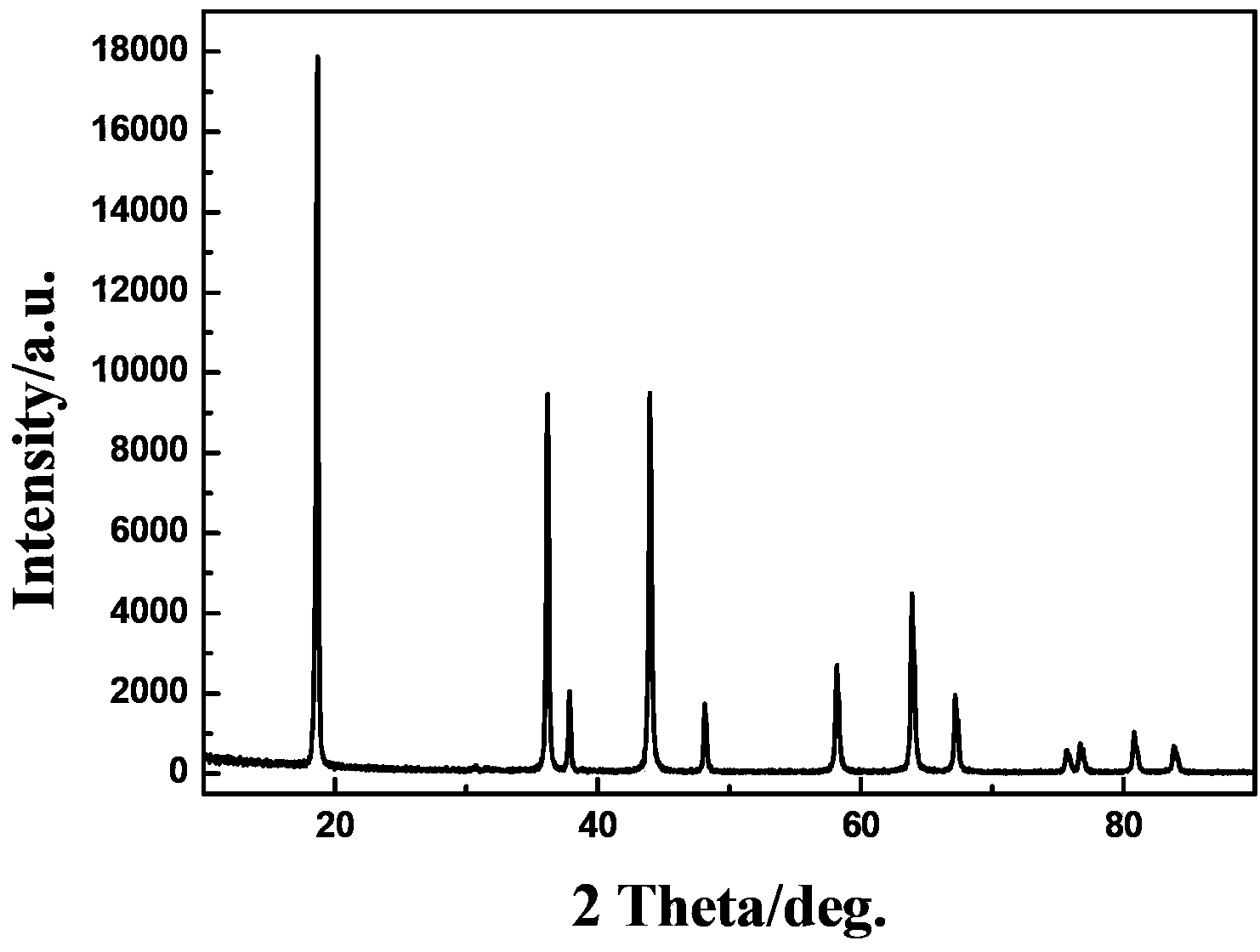
[0060] 25.1g of manganese nitrate tetrahydrate (Mn(NO 3 ) 2 ·4H 2 O) was dissolved in 1000ml of water to obtain an aqueous solution of manganese nitrate with a concentration of 0.1mol / L, and 10.6g of sodium carbonate (Na 2 CO 3 ) was dissolved in 1000 ml of water to obtain an aqueous solution of sodium carbonate with a concentration of 0.1 mol / L. Under vigorous stirring, the above-mentioned aqueous solution of sodium carbonate was poured into the aqueous solution of manganese nitrate, and after continuous stirring for 5 min, spherical manganese carbonate was obtained after suction filtration, washing and drying. Dissolve 7.938g of ammonium persulfate in 333ml of water to obtain an oxidant with a concentration of 0.1mol / L, weigh 5.75g (0.05mol) of the obtained spherical manganese carbonate and disperse it in 500ml of water, add 333ml of oxidant dropwise under vigorous stirring, and stir for 5min. Add dropwise 200 ml of hydrochloric acid with a molar concentration of 0.5 mol...
Embodiment 2
[0065] 16.9 g of manganese sulfate (MnSO 4 ·H 2 O) was dissolved in 1000ml of water to obtain an aqueous solution of manganese sulfate with a concentration of 0.1mol / L, and 138.2 potassium carbonate (K 2 CO 3 ) dissolved in 1000 ml of water to obtain an aqueous solution of potassium carbonate with a concentration of 1 mol / L. Under vigorous stirring, the above-mentioned aqueous solution of potassium carbonate was poured into the aqueous solution of manganese sulfate, and after stirring continuously for 30 min, spherical manganese carbonate was obtained after suction filtration, washing and drying. 158g potassium permanganate (KMnO 4 ) was dissolved in 1000ml of water to obtain an oxidant with a concentration of 1mol / L, weighed 11.5g (0.10mol) of spherical manganese carbonate obtained and dispersed in 1000ml of water, added 500ml of oxidant dropwise under vigorous stirring, stirred for 0.5h, and the dropwise molar concentration was 100ml of 1mol / L nitric acid, continue stirr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com